Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum?
- PMID: 23189933
- PMCID: PMC3589889
- DOI: 10.1089/ten.TEB.2012.0216
Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum?
Abstract
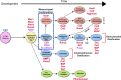
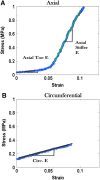
The periosteum, a thin, fibrous tissue layer covering most bones, resides in a dynamic, mechanically loaded environment. The periosteum also provides a niche for mesenchymal stem cells. The mechanics of periosteum vary greatly between species and anatomical locations, indicating the specialized role of periosteum as bone's bounding membrane. Furthermore, periosteum exhibits stress-state-dependent mechanical and material properties, hallmarks of a smart material. This review discusses what is known about the multiscale mechanical and material properties of the periosteum as well as their potential effect on the mechanosensitive progenitor cells within the tissue. Furthermore, this review addresses open questions and barriers to understanding periosteum's multiscale structure-function relationships. Knowledge of the smart material properties of the periosteum will maximize the translation of periosteum and substitute periosteum to regenerative medicine, facilitate the development of biomimetic tissue-engineered periosteum for use in instances where the native periosteum is lacking or damaged, and provide inspiration for a new class of smart, advanced materials.
Figures


References
-
- American Academy of Orthopedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Musculoskeletal Injuries. 2008. www.boneandjointburden.org/pdfs/BMUS_chpt6_injuries.pdf. www.boneandjointburden.org/pdfs/BMUS_chpt6_injuries.pdf
-
- Knothe Tate M.L. Ritzman T.F. Schneider E. Knothe U.R. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. J Bone Joint Surg Am. 2007;89:307. - PubMed
-
- Alonso J.E. Regazzoni P. Bridging bone gaps with the Ilizarov technique. Biologic principles. Clin Plast Surg. 1991;18:497. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

