Near-IR resonance Raman spectroscopy of archaerhodopsin 3: effects of transmembrane potential
- PMID: 23189985
- PMCID: PMC3568500
- DOI: 10.1021/jp309996a
Near-IR resonance Raman spectroscopy of archaerhodopsin 3: effects of transmembrane potential
Abstract
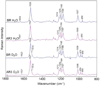
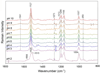
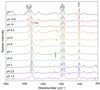
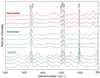
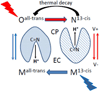
Archaerhodopsin 3 (AR3) is a light driven proton pump from Halorubrum sodomense that has been used as a genetically targetable neuronal silencer and an effective fluorescent sensor of transmembrane potential. Unlike the more extensively studied bacteriorhodopsin (BR) from Halobacterium salinarum, AR3 readily incorporates into the plasma membrane of both E. coli and mammalian cells. Here, we used near-IR resonance Raman confocal microscopy to study the effects of pH and membrane potential on the AR3 retinal chromophore structure. Measurements were performed both on AR3 reconstituted into E. coli polar lipids and in vivo in E. coli expressing AR3 in the absence and presence of a negative transmembrane potential. The retinal chromophore structure of AR3 is in an all-trans configuration almost identical to BR over the entire pH range from 3 to 11. Small changes are detected in the retinal ethylenic stretching frequency and Schiff Base (SB) hydrogen bonding strength relative to BR which may be related to a different water structure near the SB. In the case of the AR3 mutant D95N, at neutral pH an all-trans retinal O-like species (O(all-trans)) is found. At higher pH a second 13-cis retinal N-like species (N(13-cis)) is detected which is attributed to a slowly decaying intermediate in the red-light photocycle of D95N. However, the amount of N(13-cis) detected is less in E. coli cells but is restored upon addition of carbonyl cyanide m-chlorophenyl hydrazone (CCCP) or sonication, both of which dissipate the normal negative membrane potential. We postulate that these changes are due to the effect of membrane potential on the N(13-cis) to M(13-cis) levels accumulated in the D95N red-light photocycle and on a molecular level by the effects of the electric field on the protonation/deprotonation of the cytoplasmic accessible SB. This mechanism also provides a possible explanation for the observed fluorescence dependence of AR3 and other microbial rhodopsins on transmembrane potential.
Figures




Similar articles
-
Conformational changes in the archaerhodopsin-3 proton pump: detection of conserved strongly hydrogen bonded water networks.J Biol Phys. 2012 Jan;38(1):153-68. doi: 10.1007/s10867-011-9246-4. Epub 2011 Dec 10. J Biol Phys. 2012. PMID: 23277676 Free PMC article.
-
FTIR analysis of the SII540 intermediate of sensory rhodopsin II: Asp73 is the Schiff base proton acceptor.Biochemistry. 2000 Mar 21;39(11):2823-30. doi: 10.1021/bi991676d. Biochemistry. 2000. PMID: 10715101
-
Redshifted and Near-infrared Active Analog Pigments Based upon Archaerhodopsin-3.Photochem Photobiol. 2019 Jul;95(4):959-968. doi: 10.1111/php.13093. Epub 2019 Apr 8. Photochem Photobiol. 2019. PMID: 30860604 Free PMC article.
-
Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin.Biochim Biophys Acta. 2004 Jul 23;1658(1-2):72-9. doi: 10.1016/j.bbabio.2004.03.015. Biochim Biophys Acta. 2004. PMID: 15282177 Review.
-
Atomic resolution structures of bacteriorhodopsin photocycle intermediates: the role of discrete water molecules in the function of this light-driven ion pump.Biochim Biophys Acta. 2000 Aug 30;1460(1):133-56. doi: 10.1016/s0005-2728(00)00135-3. Biochim Biophys Acta. 2000. PMID: 10984596 Review.
Cited by
-
Structural Changes in an Anion Channelrhodopsin: Formation of the K and L Intermediates at 80 K.Biochemistry. 2017 Apr 25;56(16):2197-2208. doi: 10.1021/acs.biochem.7b00002. Epub 2017 Apr 10. Biochemistry. 2017. PMID: 28350445 Free PMC article.
-
Archaerhodopsin 3 is an ideal template for the engineering of highly fluorescent optogenetic reporters.Chem Sci. 2024 Nov 18;16(2):761-774. doi: 10.1039/d4sc05120c. eCollection 2025 Jan 2. Chem Sci. 2024. PMID: 39634579 Free PMC article.
-
Resonance Raman Study of an Anion Channelrhodopsin: Effects of Mutations near the Retinylidene Schiff Base.Biochemistry. 2016 Apr 26;55(16):2371-80. doi: 10.1021/acs.biochem.6b00104. Epub 2016 Apr 14. Biochemistry. 2016. PMID: 27039989 Free PMC article.
-
QuasAr Odyssey: the origin of fluorescence and its voltage sensitivity in microbial rhodopsins.Nat Commun. 2022 Sep 20;13(1):5501. doi: 10.1038/s41467-022-33084-4. Nat Commun. 2022. PMID: 36127376 Free PMC article.
-
Voltage Imaging with Engineered Proton-Pumping Rhodopsins: Insights from the Proton Transfer Pathway.ACS Phys Chem Au. 2023 May 3;3(4):320-333. doi: 10.1021/acsphyschemau.3c00003. eCollection 2023 Jul 26. ACS Phys Chem Au. 2023. PMID: 37520318 Free PMC article. Review.
References
-
- Deisseroth K. Sci. Am. 2010;303:48–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

