C-C bond formation via copper-catalyzed conjugate addition reactions to enones in water at room temperature
- PMID: 23190029
- PMCID: PMC3959802
- DOI: 10.1021/ja309409e
C-C bond formation via copper-catalyzed conjugate addition reactions to enones in water at room temperature
Abstract
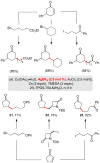
Conjugate addition reactions to enones can now be done in water at room temperature with in situ generated organocopper reagents. Mixing an enone, zinc powder, TMEDA, and an alkyl halide in a micellar environemnt containing catalytic amounts of Cu(I), Ag(I), and Au(III) leads to 1,4-adducts in good isolated yields: no organometallic precursor need be formed.
Figures



References
-
- Kharasch MS, Tawney PO. J Am Chem Soc. 1941;63:2308.
-
- Gilman H, Jones RG, Woods LA. J Org Chem. 1952;17:1630.
-
-
Erdik E. Tetrahedron. 1984;40:641.Lipshutz BH, Sengupta S. Org React. 1992;41:135.For a review on directed reactions of organocopper reagents, see: Breit B, Schmidt Y. Chem Rev. 2008;108:2928.Krause N. In: Modern Organocopper Compounds. Krause N, editor. WILEY-VCH; Weinheim: 2005. Knochel P, Yang X, Gommerman N. In: Handbook of Functionalized Organometallics Applications in Synthesis. Knochel P, editor. Vol. 2. WILEY-VCH; Weinheim: 2005. pp. 379–398.
-
-
-
For early work on Grignard- and organolithium-based cuprates, see: Normant JF. Synthesis. 1972;2:63.Corey EJ, Beames DJ. J Am Chem Soc. 1972;94:7210.Corey EJ, Katzenellenbogen JA. J Am Chem Soc. 1969;91:1851.Corey EJ, Kim CU, Chen RHK, Takeda M. J Am Chem Soc. 1972;94:4395.For more recent examples, see: Müller D, Alexakis A. Org Lett. 2012;14:1842.Tissot M, Hernández AP, Müller D, Mauduit M, Alexakis A. Org Lett. 2011;13:1524.Gremaud L, Palais L, Alexakis A. Chimia. 2012;66:196.For focused reviews on Cu-catalyzed 1,4-addition and allylic substitution reactions, see: Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéquez M. Chem Rev. 2008;108:2796.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem Rev. 2008;108:2824.
-
-
- Lipshutz BH. In: Organometallics in Synthesis A Manual. 2nd. Schlosser M, editor. John Wiley & Sons Ltd; Chichester: 2002. pp. 665–815.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

