Three-stage ex vivo expansion of high-ploidy megakaryocytic cells: toward large-scale platelet production
- PMID: 23190353
- PMCID: PMC3592379
- DOI: 10.1089/ten.TEA.2011.0111
Three-stage ex vivo expansion of high-ploidy megakaryocytic cells: toward large-scale platelet production
Abstract
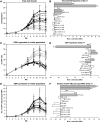
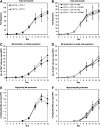
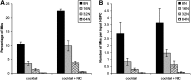
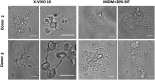
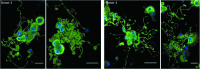
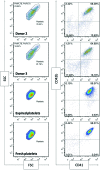
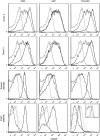
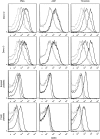
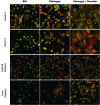
Hematopoietic stem and progenitor cells (HSPCs) have been cultured using a wide variety of cytokines to promote differentiation into megakaryocytic cells (Mks), the precursors to platelets. Greater Mk DNA content, or ploidy, has been correlated with increased platelet release. Gradients of pH, pO2, and signaling factors regulate megakaryopoiesis in the bone marrow niche. In this study, we demonstrate that a 3-phase culture process with increasing pH and pO2 and different cytokine cocktails greatly increases megakaryocyte production. CD34(+) HSPCs were first cultured at 5% O2 and pH 7.2 with a cytokine cocktail previously shown to promote Mk progenitor production. At day 5, cells were shifted to 20% O2 and pH 7.4 and maintained in 1 of 17 cytokine cocktails identified using a 2(4) factorial design of experiments method to evaluate the effects of interleukin (IL)-3, IL-6, IL-9, and high- or low-dose stem cell factor (SCF), in conjunction with thrombopoietin (Tpo) and IL-11, on expansion of mature Mks from progenitors. The combination of Tpo, high-dose SCF, IL-3, IL-9, and IL-11 best promoted Mk expansion. IL-3 greatly increased total cell fold expansion, but this was partially offset by lower Mk purity. IL-9 promoted CD41 and CD42b expression. High-dose (100 ng/mL) SCF increased Mk production and ploidy. Different commercial media and IL-3 sources substantially impacted differentiation, and X-VIVO 10 serum-free media best supported mature Mk expansion. Shifting from pH 7.4 to pH 7.6 at day 7 increased Mk production by 30%. Treatment with nicotinamide at day 7 or day 8 more than doubled the fraction of high-ploidy (>4N) Mks. Ultimately, the 3-phase culture system gave rise to 44.5±8.1 Mks and 8.5±3.1 high-ploidy Mks per input HSPC. Further optimization was required to improve platelet production. Using Iscove's modified Dulbecco's medium (IMDM)+20% BSA, insulin and transferin (BIT) 9500 Serum Substitute greatly improved the frequency and quality of Mk proplatelet extensions without affecting Mk expansion, commitment, or polyploidization in the 3-phase process. Mks cultured in IMDM+20% BIT 9500 gave rise to platelets with functional activity similar to that of fresh platelets from normal donors, as evidenced by basal tubulin distribution and the expression of surface markers and spreading in response to platelet agonists.
Figures

References
-
- Kacena M.A. Gundberg C.M. Horowitz M.C. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone. 2006;39:978. - PubMed
-
- Rafii S. Shapiro F. Pettengell R. Ferris B. Nachman R.L. Moore M.A., et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353. - PubMed
-
- Kopp H.G. Avecilla S.T. Hooper A.T. Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda, MD) 2005;20:349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

