A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism
- PMID: 23190369
- PMCID: PMC4360975
- DOI: 10.1111/gbb.12001
A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism
Abstract
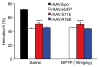
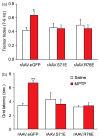
Erythropoietin (Epo) is neuroprotective in a number of preparations, but can lead to unacceptably high and even lethal hematocrit levels. Recent reports show that modified Epo variants confer neuroprotection in models of glaucoma and retinal degeneration without raising hematocrit. In this study, neuroprotective effects of two Epo variants (EpoR76E and EpoS71E) were assessed in a model of Parkinson's disease. The constructs were packaged in recombinant adeno-associated viral (rAAV) vectors and injected intramuscularly. After 3 weeks, mice received five daily injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and were killed 5 weeks later. The MPTP-lesioned mice pretreated with rAAV.eGFP (negative control) exhibited a 7- to 9-Hz tremor and slower latencies to move on a grid test (akinesia). Both of these symptomatic features were absent in mice pretreated with either modified Epo construct. The rAAV.eGFP-treated mice lesioned with MPTP exhibited a 41% reduction in tyrosine hydroxylase (TH)-positive neurons in the substantia nigra. The rAAV.EpoS71E construct did not protect nigral neurons, but neuronal loss in mice pretreated with rAAV.EpoR76E was only half that of rAAV.eGFP controls. Although dopamine levels were normal in all groups, 3,4-dihydroxyphenylacetic acid (DOPAC) was significantly reduced only in MPTP-lesioned mice pretreated with rAAV.eGFP, indicating reduced dopamine turnover. Analysis of TH-positive fibers in the striatum showed normalized density in MPTP-lesioned mice pretreated with rAAV.EpoS71E, suggesting that enhanced sprouting induced by EpoS71E may have been responsible for normal behavior and dopaminergic tone in these mice. These results show that systemically administered rAAV-generated non-erythropoietic Epo may protect against MPTP-induced parkinsonism by a combination of neuroprotection and enhanced axonal sprouting.
© 2012 The Authors. Genes, Brain and Behavior © 2012 Blackwell Publishing Ltd and International Behavioural and Neural Genetics Society.
Figures



Similar articles
-
EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: role of oxidative stress.Eur J Neurosci. 2008 Jul;28(1):41-50. doi: 10.1111/j.1460-9568.2008.06314.x. Eur J Neurosci. 2008. PMID: 18662333
-
The role of parkin in the differential susceptibility of tuberoinfundibular and nigrostriatal dopamine neurons to acute toxicant exposure.Neurotoxicology. 2015 Jan;46:1-11. doi: 10.1016/j.neuro.2014.11.004. Epub 2014 Nov 20. Neurotoxicology. 2015. PMID: 25447324 Free PMC article.
-
Evidence for a protective action of the vigilance promoting drug modafinil on the MPTP-induced degeneration of the nigrostriatal dopamine neurons in the black mouse: an immunocytochemical and biochemical analysis.Exp Brain Res. 1992;88(1):117-30. doi: 10.1007/BF02259133. Exp Brain Res. 1992. PMID: 1347270
-
Systemically administered neuregulin-1β1 rescues nigral dopaminergic neurons via the ErbB4 receptor tyrosine kinase in MPTP mouse models of Parkinson's disease.J Neurochem. 2015 May;133(4):590-7. doi: 10.1111/jnc.13026. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25581060
-
Tripchlorolide protects against MPTP-induced neurotoxicity in C57BL/6 mice.Eur J Neurosci. 2007 Sep;26(6):1500-8. doi: 10.1111/j.1460-9568.2007.05766.x. Epub 2007 Aug 20. Eur J Neurosci. 2007. PMID: 17714494
Cited by
-
Counteracting neuroinflammation in experimental Parkinson's disease favors recovery of function: effects of Er-NPCs administration.J Neuroinflammation. 2018 Nov 30;15(1):333. doi: 10.1186/s12974-018-1375-2. J Neuroinflammation. 2018. PMID: 30501635 Free PMC article.
-
Hematopoietic cytokines as therapeutic players in early stages Parkinson's disease.Front Aging Neurosci. 2015 Jul 3;7:126. doi: 10.3389/fnagi.2015.00126. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26191001 Free PMC article.
-
Targeted deletion of GD3 synthase protects against MPTP-induced neurodegeneration.Genes Brain Behav. 2017 Jun;16(5):522-536. doi: 10.1111/gbb.12377. Epub 2017 Apr 18. Genes Brain Behav. 2017. PMID: 28239983 Free PMC article.
-
Virus-mediated EpoR76E Therapy Slows Optic Nerve Axonopathy in Experimental Glaucoma.Mol Ther. 2016 Feb;24(2):230-239. doi: 10.1038/mt.2015.198. Epub 2015 Oct 27. Mol Ther. 2016. PMID: 26502777 Free PMC article.
-
Epobis is a Nonerythropoietic and Neuroprotective Agonist of the Erythropoietin Receptor with Anti-Inflammatory and Memory Enhancing Effects.Mediators Inflamm. 2016;2016:1346390. doi: 10.1155/2016/1346390. Epub 2016 Nov 21. Mediators Inflamm. 2016. PMID: 27990061 Free PMC article.
References
-
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. - PubMed
-
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. - PubMed
-
- Bezard E, Dovero S, Imbert C, Boraud T, Gross CE. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse. 2000a;38:363–368. - PubMed
-
- Bezard E, Jaber M, Gonon F, Boireau A, Bloch B, Gross CE. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Eur J Neurosci. 2000b;12:2892–2900. - PubMed
-
- Blanchet S, Marie RM, Dauvillier F, Landeau B, Benali K, Eustache F, Chavoix C. Cognitive processes involved in delayed non-matching-to-sample performance in Parkinson’s disease. Eur J Neurol. 2000;7:473–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

