Tumor-exosomes and leukocyte activation: an ambivalent crosstalk
- PMID: 23190502
- PMCID: PMC3519567
- DOI: 10.1186/1478-811X-10-37
Tumor-exosomes and leukocyte activation: an ambivalent crosstalk
Abstract
Background: Tumor-exosomes being reported to suppress or promote a cancer-directed immune response, we used exosomes of the rat pancreatic adenocarcinoma BSp73ASML (ASML) to evaluate, whether and which steps in immune response induction can be affected by tumor-exosomes and how the impaired responsiveness can be circumvented.
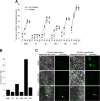
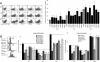
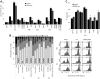
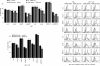
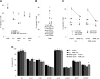
Results: ASML-exosomes bind to and are taken up by all leukocyte subpopulations in vivo and in vitro, uptake by CD11b+ leukocytes exceeding that by T and B cells. ASML-exosomes affect leukocyte proliferation via reduced CD44v6 up-regulation and lck, ZAP70 and ERK1,2 phosphorylation, which can be compensated by dendritic cells (DC). ASML-exosomes do not support Treg. Yet, impaired activation of anti-apoptotic signals is accompanied by slightly increased apoptosis susceptibility. IgM secretion is unaffected; NK and CTL activity are strengthened, ASML-exosomes co-operating with DC in CTL activation. ASML-exosomes transiently interfere with leukocyte migration by occupying migration-promoting receptors CD44, CD49d, CD62L and CD54 during binding/internalization.
Conclusion: ASML-exosomes might well serve as adjuvant in immunotherapy as they support leukocyte effector functions and have only a minor impact on leukocyte activation, which can be overridden by DC. However, exosome-induced modulation of immune cells relies, at least in part, on exosome uptake and message transfer. This implies that depending on the individual tumor's exosome composition, exosomes may distinctly affect the immune system. Nonetheless, whether immunotherapy can profit from using tumor-exosomes as adjuvant can easily be settled beforehand in vitro.
Figures

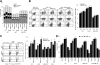
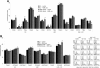
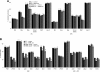

References
-
- Pap E, Pállinger E, Pásztói M, Falus A. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

