Oncogenic B-RAF(V600E) signaling induces the T-Box3 transcriptional repressor to repress E-cadherin and enhance melanoma cell invasion
- PMID: 23190890
- PMCID: PMC3788590
- DOI: 10.1038/jid.2012.421
Oncogenic B-RAF(V600E) signaling induces the T-Box3 transcriptional repressor to repress E-cadherin and enhance melanoma cell invasion
Abstract
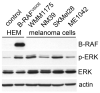
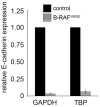
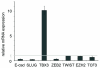
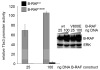
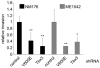
Approximately 50% of melanomas require oncogenic B-RAF(V600E) signaling for proliferation, survival, and metastasis, and the use of highly selective B-RAF inhibitors has yielded remarkable, although short-term, clinical responses. Reactivation of signaling downstream of B-RAF is frequently associated with acquired resistance to B-RAF inhibitors, and the identification of B-RAF targets may therefore provide new strategies for managing melanoma. In this report, we applied whole-genome expression analyses to reveal that oncogenic B-RAF(V600E) regulates genes associated with epithelial-mesenchymal transition in normal cutaneous human melanocytes. Most prominent was the B-RAF-mediated transcriptional repression of E-cadherin, a keratinocyte-melanoma adhesion molecule whose loss is intimately associated with melanoma invasion and metastasis. Here we identify a link between oncogenic B-RAF, the transcriptional repressor Tbx3, and E-cadherin. We show that B-RAF(V600E) induces the expression of Tbx3, which potently represses E-cadherin expression in melanocytes and melanoma cells. Tbx3 expression is normally restricted to developmental embryonic tissues and promoting cell motility, but it is also aberrantly increased in various cancers and has been linked to tumor cell invasion and metastasis. We propose that this B-RAF/Tbx3/E-cadherin pathway has a critical role in promoting the metastasis of B-RAF-mutant melanomas.
Figures






Comment in
-
Mutant BRAF: a novel mediator of microenvironmental escape in melanoma?J Invest Dermatol. 2013 May;133(5):1135-7. doi: 10.1038/jid.2012.474. J Invest Dermatol. 2013. PMID: 23594535
References
-
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the american joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. - PubMed
-
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. - PubMed
-
- Basolo F, Torregrossa L, Giannini R, Miccoli M, Lupi C, Sensi E, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. - PubMed
-
- Becker TM, Philipsz S, Scurr LL, Fung C, Haferkamp S, Kefford RF, et al. Oncogenic B-RAF(V600E) promotes anchorage-independent survival of human melanocytes. J Invest Dermatol. 2010;130:2144–2147. - PubMed
-
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

