Genetic characterization of a reptilian calicivirus (Cro1)
- PMID: 23190937
- PMCID: PMC3519611
- DOI: 10.1186/1743-422X-9-297
Genetic characterization of a reptilian calicivirus (Cro1)
Abstract
Background: Vesiviruses in the family Caliciviridae infect a broad range of animal hosts including mammals, birds, fish, amphibians and reptiles. The vesivirus Cro1 strains were isolated from diseased snakes in the San Diego zoo in 1978 and reported as the first caliciviruses found in reptiles. The goal of this study was to characterize the Cro1 strain 780032I that was isolated in cell culture from a rock rattlesnake (Crotalus lepidus) in the original outbreak.
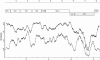
Results: We re-amplified the original virus stock in Vero cells, and determined its full-length genome sequence. The Cro1 genome is 8296 nucleotides (nt) in length and has a typical vesivirus organization, with three open reading frames (ORF), ORF1 (5643 nt), ORF2 (2121 nt), and ORF3 (348 nt) encoding a nonstructural polyprotein, the major capsid protein precursor, and a minor structural protein, respectively. Phylogenetic analysis of the full-length genome sequence revealed that the Cro1 virus clustered most closely with the VESV species of the genus Vesivirus, but was genetically distinct (82-83% identities with closest strains).
Conclusions: This is the first description of a full-length genome sequence from a reptile calicivirus (Cro1). The availability of the Cro1 genome sequence should facilitate investigation of the molecular mechanisms involved in Cro1 virus evolution and host range.
Figures
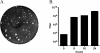

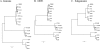

References
-
- Green KY. Caliciviridae: The Noroviruses. PA, Lippincott Williams & Wilkins, Philadelphia; 2007. pp. 949–979. (Fields Virology. Volume 1). [Knipe DM HP (Series Editor)
-
- Clarke IN, Lambden PR. Organization and expression of calicivirus genes. J Infect Dis. 2000;181(Suppl 2):S309–S316. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

