Crystallization and preliminary X-ray data analysis of the pXO1 plasmid-partitioning factor TubZ from Bacillus cereus
- PMID: 23192045
- PMCID: PMC3509986
- DOI: 10.1107/S1744309112045551
Crystallization and preliminary X-ray data analysis of the pXO1 plasmid-partitioning factor TubZ from Bacillus cereus
Abstract
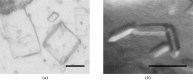
TubZ is a structural homologue of tubulin and FtsZ GTPases, which are involved in the type III plasmid-partitioning system. TubZ assembles into polymers in a GTP-dependent manner and drives plasmid segregation as `cytomotive' filaments. In this study, C-terminally truncated TubZ from Bacillus cereus was crystallized in the presence or absence of GDP by the hanging-drop vapour-diffusion method. The crystal of TubZ in complex with GDP belonged to the monoclinic space group P2(1), with unit-cell parameters a=67.05, b=84.49, c=67.66 Å, β=92.92°, and was non-isomorphous with GDP-bound TubZ previously crystallized in the presence of the slowly hydrolysable GTP analogue GTPγS. TubZ was also crystallized in the free form and the crystal belonged to space group P2(1), with unit-cell parameters a=53.91, b=65.54, c=58.18 Å, β=106.19°. Data were collected to 1.7 and 2.1 Å resolution for the free and GDP-bound forms, respectively.
Figures

Similar articles
-
Filament formation of the FtsZ/tubulin-like protein TubZ from the Bacillus cereus pXO1 plasmid.J Biol Chem. 2012 Sep 14;287(38):32103-12. doi: 10.1074/jbc.M112.373803. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22847006 Free PMC article.
-
Cooperative DNA Binding of the Plasmid Partitioning Protein TubR from the Bacillus cereus pXO1 Plasmid.J Mol Biol. 2018 Dec 7;430(24):5015-5028. doi: 10.1016/j.jmb.2018.11.001. Epub 2018 Nov 8. J Mol Biol. 2018. PMID: 30414406
-
In vitro assembly studies of FtsZ/tubulin-like proteins (TubZ) from Bacillus plasmids: evidence for a capping mechanism.J Biol Chem. 2008 Mar 28;283(13):8102-9. doi: 10.1074/jbc.M709163200. Epub 2008 Jan 15. J Biol Chem. 2008. PMID: 18198178 Free PMC article.
-
Tubulin-Like Proteins in Prokaryotic DNA Positioning.Subcell Biochem. 2017;84:323-356. doi: 10.1007/978-3-319-53047-5_11. Subcell Biochem. 2017. PMID: 28500531 Review.
-
Tubulin and FtsZ form a distinct family of GTPases.Nat Struct Biol. 1998 Jun;5(6):451-8. doi: 10.1038/nsb0698-451. Nat Struct Biol. 1998. PMID: 9628483 Review.
Cited by
-
The IntXO-PSL Recombination System Is a Key Component of the Second Maintenance System for Bacillus anthracis Plasmid pXO1.J Bacteriol. 2016 Jun 27;198(14):1939-1951. doi: 10.1128/JB.01004-15. Print 2016 Jul 15. J Bacteriol. 2016. PMID: 27137503 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

