Production, purification, crystallization and structure determination of H-1 Parvovirus
- PMID: 23192051
- PMCID: PMC3509992
- DOI: 10.1107/S1744309112045563
Production, purification, crystallization and structure determination of H-1 Parvovirus
Abstract
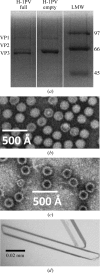
Crystals of H-1 Parvovirus (H-1PV), an antitumor gene-delivery vector, were obtained for DNA-containing capsids and diffracted X-rays to 2.7 Å resolution using synchrotron radiation. The crystals belonged to the monoclinic space group P2(1), with unit-cell parameters a=255.4, b=350.4, c=271.6 Å, β=90.34°. The unit cell contained two capsids, with one capsid per crystallographic asymmetric unit. The H-1PV structure has been determined by molecular replacement and is currently being refined.
Figures

Similar articles
-
Structural characterization of H-1 parvovirus: comparison of infectious virions to empty capsids.J Virol. 2013 May;87(9):5128-40. doi: 10.1128/JVI.03416-12. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449783 Free PMC article.
-
Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 9.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009 Jul 1;65(Pt 7):715-8. doi: 10.1107/S1744309109021460. Epub 2009 Jun 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009. PMID: 19574648 Free PMC article.
-
Production, purification, crystallization and preliminary X-ray analysis of adeno-associated virus serotype 8.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Jun 1;61(Pt 6):558-61. doi: 10.1107/S1744309105014132. Epub 2005 Jun 1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005. PMID: 16511095 Free PMC article.
-
Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 7.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Dec 1;63(Pt 12):1073-6. doi: 10.1107/S1744309107060289. Epub 2007 Nov 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 18084098 Free PMC article.
-
Crystallization of RNA.Cell Mol Life Sci. 2001 Feb;58(2):234-43. doi: 10.1007/PL00000851. Cell Mol Life Sci. 2001. PMID: 11289305 Free PMC article. Review.
Cited by
-
Structural characterization of H-1 parvovirus: comparison of infectious virions to empty capsids.J Virol. 2013 May;87(9):5128-40. doi: 10.1128/JVI.03416-12. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449783 Free PMC article.
-
A novel scalable, robust downstream process for oncolytic rat parvovirus: isoelectric point-based elimination of empty particles.Appl Microbiol Biotechnol. 2017 Apr;101(8):3143-3152. doi: 10.1007/s00253-016-8071-x. Epub 2017 Jan 14. Appl Microbiol Biotechnol. 2017. PMID: 28091791 Free PMC article.
-
Structure of neurotropic adeno-associated virus AAVrh.8.J Struct Biol. 2015 Oct;192(1):21-36. doi: 10.1016/j.jsb.2015.08.017. Epub 2015 Aug 31. J Struct Biol. 2015. PMID: 26334681 Free PMC article.
-
Parvoviruses cause nuclear envelope breakdown by activating key enzymes of mitosis.PLoS Pathog. 2013 Oct;9(10):e1003671. doi: 10.1371/journal.ppat.1003671. Epub 2013 Oct 31. PLoS Pathog. 2013. PMID: 24204256 Free PMC article.
-
Stability and safety key factors of the oncolytic protoparvovirus H-1 from manufacturing to human application.Appl Microbiol Biotechnol. 2023 Aug;107(15):4777-4787. doi: 10.1007/s00253-023-12521-4. Epub 2023 May 20. Appl Microbiol Biotechnol. 2023. PMID: 37209160 Free PMC article.
References
-
- Agbandje, M., McKenna, R., Rossmann, M. G., Strassheim, M. L. & Parrish, C. R. (1993). Proteins, 16, 155–171. - PubMed
-
- Agbandje-McKenna, M. & Chapman, M. S. (2006). Parvoviruses, edited by J. R. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden & C. R. Parrish, pp. 125–139. London: Hodder Arnold.
-
- Agbandje-McKenna, M., Llamas-Saiz, A. L., Wang, F., Tattersall, P. & Rossmann, M. G. (1998). Structure, 6, 1369–1381. - PubMed
-
- Angelova, A. L., Aprahamian, M., Grekova, S. P., Hajri, A., Leuchs, B., Giese, N. A., Dinsart, C., Herrmann, A., Balboni, G., Rommelaere, J. & Raykov, Z. (2009). Clin. Cancer Res. 15, 511–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

