Studies on the interaction mechanism of pyrene derivatives with human tumor-related DNA
- PMID: 23192191
- PMCID: PMC6268327
- DOI: 10.3390/molecules171214159
Studies on the interaction mechanism of pyrene derivatives with human tumor-related DNA
Abstract
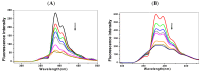
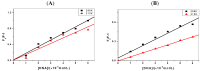
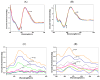
Pyrene derivatives can be carcinogenic, teratogenic and mutagenic, thus having the potential to cause malignant diseases. In this work, the interactions of two selected pyrene derivatives (1-OHP and 1-PBO) and human tumor-related DNA (p53 DNA and C-myc DNA) are investigated by spectroscopic and non-native polyacrylamide gel electrophoresis (PAGE) methods. Using fluorescence spectrometry and circular dichroism (CD), DNA interactions of pyrene derivatives are confirmed to occur mainly via the groove binding mode supported by the intercalation into the base pairs of DNA. There is an obvious binding order of pyrene derivatives to the targeted DNA, 1-OHP > 1-PBO. The binding constants of 1-OHP are 1.16 × 10(6) L × mol(-1) and 4.04 × 10(5) L × mol(-1) for p53 DNA and C-myc DNA, respectively, while that of 1-PBO are only 2.04 × 10(3) L × mol(-1) and 1.39 × 10(3) L × mol(-1) for p53 DNA and C-myc DNA, respectively. Besides, the binding of pyrene derivatives to p53 DNA is stronger than that for C-myc DNA. CD and PAGE results indicate that the binding of pyrene derivatives can affect the helical structures of DNA and further induce the formation of double-chain antiparallel G-quadruplex DNA of hybrid G-rich sequences.
Figures


References
-
- Øvrevik J., Arlt V.M., Øya E., Nagy E., Mollerup S., Phillips D.H., Låg M., Holme J.A. Differential effects of nitro-PAHs and amino-PAHs on cytokine and chemokine responses in human bronchial epithelial BEAS-2B cells. Toxicol. Appl. Pharm. 2010;242:70–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

