Asymmetric ion-pairing catalysis
- PMID: 23192886
- PMCID: PMC4284951
- DOI: 10.1002/anie.201205449
Asymmetric ion-pairing catalysis
Abstract
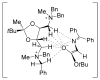
Charged intermediates and reagents are ubiquitous in organic transformations. The interaction of these ionic species with chiral neutral, anionic, or cationic small molecules has emerged as a powerful strategy for catalytic, enantioselective synthesis. This review describes developments in the burgeoning field of asymmetric ion-pairing catalysis with an emphasis on the insights that have been gleaned into the structural and mechanistic features that contribute to high asymmetric induction.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


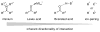


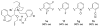





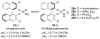

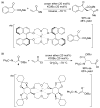


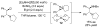
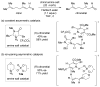
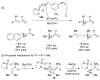




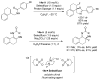

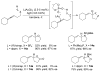
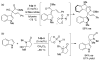

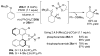

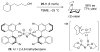

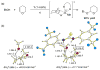
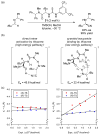

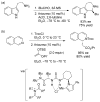
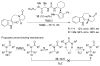
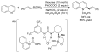
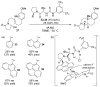
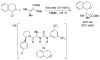
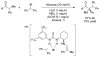
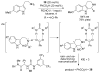
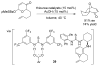
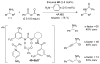
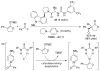
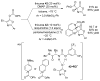
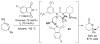
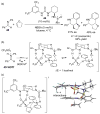
References
-
- Bjerrum N. K Dan Vldesk Selesk Math Phys Medd. 1926;7:3.
- Szwarc M. Acc Chem Res. 1969;2:87–96.
-
- Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito: 2006.
-
- Winstein S, Clippinger E, Fainberg AH, Robinson GC. J Am Chem Soc. 1954;76:2597–2598.
- Sadek H, Fuoss RM. J Am Chem Soc. 1954;76:5897–5901.
-
- O’Donnell MJ. Acc Chem Res. 2004;37:506–517. - PubMed
- Lygo B, Andrews BI. Acc Chem Res. 2004;37:518–525. - PubMed
- Hashimoto T, Maruoka K. Chem Rev. 2007;107:5656–5682. - PubMed
- Ooi T, Maruoka K. Angew Chem. 2007;119:4300–4345.
- Angew Chem Int Ed. 2007;46:4222–4266. - PubMed
- Jew S-s, Park H-g. Chem Commun. 2009:7090–7103. - PubMed
- Maruoka K. Asymmetric Phase Transfer Catalysis. Wiley-VCH; New York: 2008.
- Ojima I. Catalytic Asymmetric Synthesis. 3. Wiley-VCH; New York: 2010.
- Ooi T, Maruoka K. Acc Chem Res. 2004;37:526–533. - PubMed
- Dolling UH, Davis P, Grabowski EJJ. J Am Chem Soc. 1984;106:446–447.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

