Insulin inhibits cardiac mesoderm, not mesendoderm, formation during cardiac differentiation of human pluripotent stem cells and modulation of canonical Wnt signaling can rescue this inhibition
- PMID: 23193013
- PMCID: PMC3582800
- DOI: 10.1002/stem.1289
Insulin inhibits cardiac mesoderm, not mesendoderm, formation during cardiac differentiation of human pluripotent stem cells and modulation of canonical Wnt signaling can rescue this inhibition
Abstract
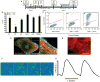
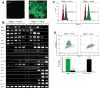
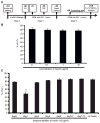
The study of the regulatory signaling hierarchies of human heart development is limited by a lack of model systems that can reproduce the precise developmental events that occur during human embryogenesis. The advent of human pluripotent stem cell (hPSC) technology and robust cardiac differentiation methods affords a unique opportunity to monitor the full course of cardiac induction in vitro. Here, we show that stage-specific activation of insulin signaling strongly inhibited cardiac differentiation during a monolayer-based differentiation protocol that used transforming growth factor β superfamily ligands to generate cardiomyocytes. However, insulin did not repress cardiomyocyte differentiation in a defined protocol that used small molecule regulators of canonical Wnt signaling. By examining the context of insulin inhibition of cardiomyocyte differentiation, we determined that the inhibitory effects by insulin required Wnt/β-catenin signaling and that the cardiomyocyte differentiation defect resulting from insulin exposure was rescued by inhibition of Wnt/β-catenin during the cardiac mesoderm (Nkx2.5+) stage. Thus, insulin and Wnt/β-catenin signaling pathways, as a network, coordinate to influence hPSC differentiation to cardiomyocytes, with the Wnt/β-catenin pathway dominant to the insulin pathway. Our study contributes to the understanding of the regulatory hierarchies of human cardiomyocyte differentiation and has implications for modeling human heart development.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing. All the other authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

