PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant
- PMID: 23193190
- PMCID: PMC3581188
- DOI: 10.2337/db12-0929
PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant
Abstract
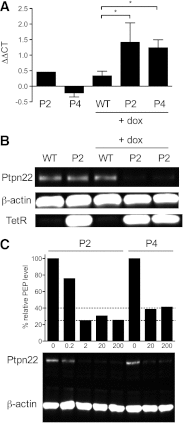
PTPN22 encodes the lymphoid tyrosine phosphatase (LYP) and is the second strongest non-HLA genetic risk factor for type 1 diabetes. The PTPN22 susceptibility allele generates an LYP variant with an arginine-to-tryptophan substitution at position 620 (R620W) that has been reported by several studies to impart a gain of function. However, a recent report investigating both human cells and a knockin mouse model containing the R620W homolog suggested that this variation causes faster protein degradation. Whether LYP R620W is a gain- or loss-of-function variant, therefore, remains controversial. To address this issue, we generated transgenic NOD mice (nonobese diabetic) in which Ptpn22 can be inducibly silenced by RNA interference. We found that Ptpn22 silencing in the NOD model replicated many of the phenotypes observed in C57BL/6 Ptpn22 knockout mice, including an increase in regulatory T cells. Notably, loss of Ptpn22 led to phenotypic changes in B cells opposite to those reported for the human susceptibility allele. Furthermore, Ptpn22 knockdown did not increase the risk of autoimmune diabetes but, rather, conferred protection from disease. Overall, to our knowledge, this is the first functional study of Ptpn22 within a model of type 1 diabetes, and the data do not support a loss of function for the PTPN22 disease variant.
Figures


Similar articles
-
Overexpression of the PTPN22 Autoimmune Risk Variant LYP-620W Fails to Restrain Human CD4+ T Cell Activation.J Immunol. 2021 Aug 1;207(3):849-859. doi: 10.4049/jimmunol.2000708. Epub 2021 Jul 23. J Immunol. 2021. PMID: 34301848 Free PMC article.
-
Ptpn22 and Cd2 Variations Are Associated with Altered Protein Expression and Susceptibility to Type 1 Diabetes in Nonobese Diabetic Mice.J Immunol. 2015 Nov 15;195(10):4841-52. doi: 10.4049/jimmunol.1402654. Epub 2015 Oct 5. J Immunol. 2015. PMID: 26438525 Free PMC article.
-
Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice.J Immunol. 2013 Jul 15;191(2):594-607. doi: 10.4049/jimmunol.1203380. Epub 2013 Jun 10. J Immunol. 2013. PMID: 23752610
-
The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity.Autoimmun Rev. 2013 May;12(7):717-25. doi: 10.1016/j.autrev.2012.12.003. Epub 2012 Dec 20. Autoimmun Rev. 2013. PMID: 23261816 Review.
-
The potential of PTPN22 as a therapeutic target for rheumatoid arthritis.Expert Opin Ther Targets. 2018 Oct;22(10):879-891. doi: 10.1080/14728222.2018.1526924. Expert Opin Ther Targets. 2018. PMID: 30251905 Review.
Cited by
-
Lack of the protein tyrosine phosphatase PTPN22 strengthens transplant tolerance to pancreatic islets in mice.Diabetologia. 2015 Jun;58(6):1319-28. doi: 10.1007/s00125-015-3540-9. Epub 2015 Mar 7. Diabetologia. 2015. PMID: 25748328
-
Experimental colitis in IL-10-deficient mice ameliorates in the absence of PTPN22.Clin Exp Immunol. 2019 Sep;197(3):263-275. doi: 10.1111/cei.13339. Epub 2019 Jul 10. Clin Exp Immunol. 2019. PMID: 31194881 Free PMC article.
-
Comparative genetics: synergizing human and NOD mouse studies for identifying genetic causation of type 1 diabetes.Rev Diabet Stud. 2012 Winter;9(4):169-87. doi: 10.1900/RDS.2012.9.169. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804259 Free PMC article. Review.
-
Genetic analysis of type 1 diabetes: embryonic stem cells as new tools to unlock biological mechanisms in type 1 diabetes.Rev Diabet Stud. 2012 Winter;9(4):137-47. doi: 10.1900/RDS.2012.9.137. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804257 Free PMC article. Review.
-
Distribution of PTPN22 polymorphisms in SLE from western Mexico: correlation with mRNA expression and disease activity.Clin Exp Med. 2016 Aug;16(3):399-406. doi: 10.1007/s10238-015-0359-0. Epub 2015 May 27. Clin Exp Med. 2016. PMID: 26013387
References
-
- Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood 1999;93:2013–2024 - PubMed
-
- Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol 2007;179:4704–4710 - PubMed
-
- Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004;36:337–338 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

