Clinical evaluation of a personalized artificial pancreas
- PMID: 23193210
- PMCID: PMC3609541
- DOI: 10.2337/dc12-0948
Clinical evaluation of a personalized artificial pancreas
Abstract
Objective: An artificial pancreas (AP) that automatically regulates blood glucose would greatly improve the lives of individuals with diabetes. Such a device would prevent hypo- and hyperglycemia along with associated long- and short-term complications as well as ease some of the day-to-day burden of frequent blood glucose measurements and insulin administration.
Research design and methods: We conducted a pilot clinical trial evaluating an individualized, fully automated AP using commercial devices. Two trials (n = 22, n(subjects) = 17) were conducted using a multiparametric formulation of model predictive control and an insulin-on-board algorithm such that the control algorithm, or "brain," can be embedded on a chip as part of a future mobile device. The protocol evaluated the control algorithm for three main challenges: 1) normalizing glycemia from various initial glucose levels, 2) maintaining euglycemia, and 3) overcoming an unannounced meal of 30 ± 5 g carbohydrates.
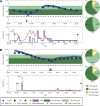
Results: Initial glucose values ranged from 84-251 mg/dL. Blood glucose was kept in the near-normal range (80-180 mg/dL) for an average of 70% of the trial time. The low and high blood glucose indices were 0.34 and 5.1, respectively.
Conclusions: These encouraging short-term results reveal the ability of a control algorithm tailored to an individual's glucose characteristics to successfully regulate glycemia, even when faced with unannounced meals or initial hyperglycemia. To our knowledge, this represents the first truly fully automated multiparametric model predictive control algorithm with insulin-on-board that does not rely on user intervention to regulate blood glucose in individuals with type 1 diabetes.
Figures

References
-
- Bellazzi R, Arcelloni M, Bensa G, et al. Design, methods, and evaluation directions of a multi-access service for the management of diabetes mellitus patients. Diabetes Technol Ther 2003;5:621–629 - PubMed
-
- Rebrin K, Steil GM, Panteleon AE, Hariri F, Darwin C, Saad MF. Closed-Loop subcutaneous insulin delivery based on subcutaneous glucose sensing in adults. Diabetologia 2004;47:A93
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

