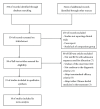
Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials
- PMID: 23193426
- PMCID: PMC3491750
- DOI: 10.1155/2012/630124
Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials
Abstract
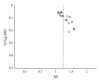
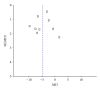
Buyang Huanwu Decoction (BHD) is a well-known traditional Chinese herbal prescription for treating stroke-induced disability. The objective of this study was to evaluate the efficacy and safety of BHD for acute ischemic stroke. A systematic literature search was performed in 6 databases until February 2012. Randomized controlled clinical trials (RCTs) that evaluate efficacy and safety of BHD for acute ischemic stroke were included. Nineteen RCTs with 1580 individuals were identified. The studies were generally of low methodological quality. Only one of the trial included death or dependency as a primary outcome measure. Only 4 trials reported adverse events. Meta-analysis showed the clinical effective rate of neurological deficit improvement favoring BHD when compared with western conventional medicines (WCM), P < 0.001. There is significant difference in the neurologic deficit score between the BHD treatment group and the WCM control group, P < 0.001. In Conclusion, BHD appears to improve neurological deficit and seems generally safe in patients with acute ischemic stroke. However, the current evidence is insufficient to support a routine use of BHD for acute ischemic stroke due to the poor methodological quality and lack of adequate safety data of the included studies. Further rigorously designed trials are required.
Figures
References
-
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. The Lancet Neurology. 2003;2(1):43–53. - PubMed
-
- World Health Organization. STEPwise approach to stroke surveillance. http://www.who.int/chp/steps/stroke/en/index.html.
-
- Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. The Lancet Neurology. 2009;8(4):345–354. - PubMed
-
- Panagos PD. The approach to optimizing stroke care. The American Journal of Emergency Medicine. 2008;26(7):808–816. - PubMed
LinkOut - more resources
Full Text Sources