Treatment with the cytochrome P450 ω-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats
- PMID: 23194285
- PMCID: PMC3623058
- DOI: 10.1111/bph.12079
Treatment with the cytochrome P450 ω-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats
Abstract
Background and purpose: Hypertension increases cerebrovascular oxidative stress and inflammation and impairs vasomotor function. These pathological alterations lead to dysregulation of cerebral blood flow and exacerbate atherogenesis, increasing the morbidity of ischaemic cerebrovascular diseases and promoting vascular cognitive impairment. We aimed to test the hypothesis that increased production of the arachidonic acid metabolite 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) contributes to hypertension-induced cerebrovascular alterations.
Experimental approach: We treated male spontaneously hypertensive rats (SHR) with HET0016 (N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine), an inhibitor of 20-HETE synthesis. In middle cerebral arteries (MCAs) of SHRs, we focused on vasomotor responses and end points that are highly relevant for cellular reactive oxygen species (ROS) production, inflammatory cytokine expression and NF-κB activation.
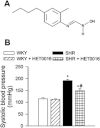
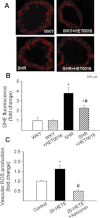
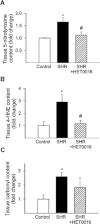
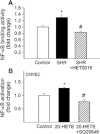
Key results: SHRs treated with HET0016 remained hypertensive (SHR + HET0016: 149 ± 8 mmHg, Wistar-Kyoto rat: 115 ± 4 mmHg; P < 0.05.), although their systolic blood pressure was decreased compared to untreated SHRs (191 ± 6 mmHg). In MCAs of SHRs, flow-induced constriction was increased, whereas ACh- and ATP-induced dilations were impaired. This functional impairment was reversed by treatment with HET0016. Treatment with HET0016 also significantly decreased oxidative stress in MCAs of SHRs (as shown by dihydroethidium staining and analysis of vascular 5-nitrotyrosine, 4-hydroxynonenal and carbonyl content) and inhibited cerebrovascular inflammation (shown by the reduced mRNA expression of TNFα, IL-1β and IL-6). Treatment of SHRs with HET0016 also attenuated vascular NF-κB activation. In vitro treatment with 20-HETE significantly increased vascular production of ROS and promoted NF-κB activation in cultured cerebromicrovascular endothelial cells.
Conclusions and implications: Taken together, treatment with HET0016 confers anti-oxidative and anti-inflammatory effects in the cerebral arteries of SHRs by disrupting 20-HETE-mediated autocrine/paracrine signalling pathways in the vascular wall. It is likely that HET0016-induced decreases in blood pressure also potentiate the cerebrovascular protective effects of the drug.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

References
-
- Aiyagari V, Gorelick PB. Management of blood pressure for acute and recurrent stroke. Stroke. 2009;40:2251–2256. - PubMed
-
- Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. - PubMed
-
- Amenta F, Di Tullio MA, Tomassoni D. Arterial hypertension and brain damage – evidence from animal models (review) Clin Exp Hypertens. 2003;25:359–380. - PubMed
-
- Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

