Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L.)
- PMID: 23194296
- PMCID: PMC3521190
- DOI: 10.1186/1472-6750-12-92
Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L.)
Abstract
Background: Transferrin (TF) plays a critical physiological role in cellular iron delivery via the transferrin receptor (TFR)-mediated endocytosis pathway in nearly all eukaryotic organisms. Human serum TF (hTF) is extensively used as an iron-delivery vehicle in various mammalian cell cultures for production of therapeutic proteins, and is also being explored for use as a drug carrier to treat a number of diseases by employing its unique TFR-mediated endocytosis pathway. With the increasing concerns over the risk of transmission of infectious pathogenic agents of human plasma-derived TF, recombinant hTF is preferred to use for these applications. Here, we carry out comparative studies of the TFR binding, TFR-mediated endocytosis and cellular iron delivery of recombinant hTF from rice (rhTF), and evaluate its suitability for biopharmaceutical applications.
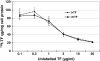
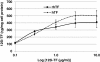
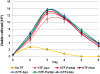
Result: Through a TFR competition binding affinity assay with HeLa human cervic carcinoma cells (CCL-2) and Caco-2 human colon carcinoma cells (HTB-37), we show that rhTF competes similarly as hTF to bind TFR, and both the TFR binding capacity and dissociation constant of rhTF are comparable to that of hTF. The endocytosis assay confirms that rhTF behaves similarly as hTF in the slow accumulation in enterocyte-like Caco-2 cells and the rapid recycling pathway in HeLa cells. The pulse-chase assay of rhTF in Caco-2 and HeLa cells further illustrates that rice-derived rhTF possesses the similar endocytosis and intracellular processing compared to hTF. The cell culture assays show that rhTF is functionally similar to hTF in the delivery of iron to two diverse mammalian cell lines, HL-60 human promyelocytic leukemia cells (CCL-240) and murine hybridoma cells derived from a Sp2/0-Ag14 myeloma fusion partner (HB-72), for supporting their proliferation, differentiation, and physiological function of antibody production.
Conclusion: The functional similarity between rice derived rhTF and native hTF in their cellular iron delivery, TFR binding, and TFR-mediated endocytosis and intracellular processing support that rice-derived rhTF can be used as a safe and animal-free alternative to serum hTF for bioprocessing and biopharmaceutical applications.
Figures





References
-
- He QY, Mason A. In: Molecular and Cellular Iron Transport, CRC. Templeton DM, editor. 2002. Molecular aspects of release of iron from transferrin; pp. 95–124.
-
- Mortellaro S, Devine M. Advance in animal-free manufacturing of biopharmaceuticals. Biopharm Int. 2007;20(Supp):30–37.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

