Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond
- PMID: 23194300
- PMCID: PMC3526508
- DOI: 10.1186/1471-2172-13-63
Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond
Abstract
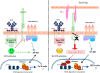
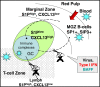
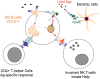
The immunological roles of B-cells are being revealed as increasingly complex by functions that are largely beyond their commitment to differentiate into plasma cells and produce antibodies, the key molecular protagonists of innate immunity, and also by their compartmentalisation, a more recently acknowledged property of this immune cell category. For decades, B-cells have been recognised by their expression of an immunoglobulin that serves the function of an antigen receptor, which mediates intracellular signalling assisted by companion molecules. As such, B-cells were considered simple in their functioning compared to the other major type of immune cell, the T-lymphocytes, which comprise conventional T-lymphocyte subsets with seminal roles in homeostasis and pathology, and non-conventional T-lymphocyte subsets for which increasing knowledge is accumulating. Since the discovery that the B-cell family included two distinct categories - the non-conventional, or extrafollicular, B1 cells, that have mainly been characterised in the mouse; and the conventional, or lymph node type, B2 cells - plus the detailed description of the main B-cell regulator, FcγRIIb, and the function of CD40(+) antigen presenting cells as committed/memory B-cells, progress in B-cell physiology has been slower than in other areas of immunology. Cellular and molecular tools have enabled the revival of innate immunity by allowing almost all aspects of cellular immunology to be re-visited. As such, B-cells were found to express "Pathogen Recognition Receptors" such as TLRs, and use them in concert with B-cell signalling during innate and adaptive immunity. An era of B-cell phenotypic and functional analysis thus began that encompassed the study of B-cell microanatomy principally in the lymph nodes, spleen and mucosae. The novel discovery of the differential localisation of B-cells with distinct phenotypes and functions revealed the compartmentalisation of B-cells. This review thus aims to describe novel findings regarding the B-cell compartments found in the mouse as a model organism, and in human physiology and pathology. It must be emphasised that some differences are noticeable between the mouse and human systems, thus increasing the complexity of B-cell compartmentalisation. Special attention will be given to the (lymph node and spleen) marginal zones, which represent major crossroads for B-cell types and functions and a challenge for understanding better the role of B-cell specificities in innate and adaptive immunology.
Figures

References
-
- Zouali M. B lymphocytes–chief players and therapeutic targets in autoimmune diseases. Front Biosci. 2008;13:4852–4861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

