Age sensitizes the kidney to heme protein-induced acute kidney injury
- PMID: 23195679
- PMCID: PMC3566520
- DOI: 10.1152/ajprenal.00606.2012
Age sensitizes the kidney to heme protein-induced acute kidney injury
Abstract
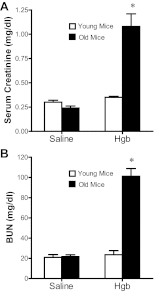
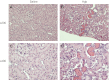
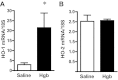
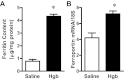
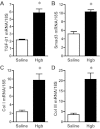
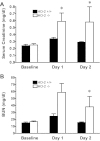
Age increases the risk for ischemic acute kidney injury (AKI). We questioned whether a similar age-dependent injury occurs following exposure to hemoglobin, a known nephrotoxin. Old mice (~16 mo old), but not young mice (~6 mo old), when administered hemoglobin, exhibited marked elevation in blood urea nitrogen (BUN) and serum creatinine, and acute tubular necrosis with prominent tubular cast formation. The aged kidney exhibited induction of heme oxygenase-1 (HO-1) and other genes/proteins that may protect against heme-mediated renal injury, including ferritin, ferroportin, haptoglobin, and hemopexin. Old mice did not evince induction of HO-2 mRNA by hemoglobin, whereas a modest induction of HO-2 mRNA was observed in young mice. To determine the functional significance of HO-2 in heme protein-induced AKI, we administered hemoglobin to relatively young HO-2(+/+) and HO-2(-/-) mice: HO-2(-/-) mice, compared with HO-2(+/+) mice, exhibited greater renal dysfunction and histologic injury when administered hemoglobin. In addition to failing to elicit a protective system such as HO-2 in response to hemoglobin, old mice exhibited an exaggerated maladaptive response typified by markedly greater induction of the nephrotoxic cytokine IL-6 (130-fold increase vs. 10-fold increase in mRNA in young mice). We conclude that aged mice, unlike relatively younger mice, are exquisitely sensitive to the nephrotoxicity of hemoglobin, an effect attended by a failure to induce HO-2 mRNA and a fulminant upregulation of IL-6. Age thus markedly augments the sensitivity of the kidney to heme proteins, and HO-2 confers resistance to such insults.
Figures

Comment in
-
Aging and hemoglobin-induced acute kidney injury.Am J Physiol Renal Physiol. 2013 May 1;304(9):F1167-8. doi: 10.1152/ajprenal.00032.2013. Epub 2013 Jan 30. Am J Physiol Renal Physiol. 2013. PMID: 23364802 Free PMC article. No abstract available.
Similar articles
-
The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo.Am J Pathol. 2000 May;156(5):1527-35. doi: 10.1016/S0002-9440(10)65024-9. Am J Pathol. 2000. PMID: 10793064 Free PMC article.
-
Heme oxygenase-2 protects against ischemic acute kidney injury: influence of age and sex.Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F695-F704. doi: 10.1152/ajprenal.00085.2019. Epub 2019 Jun 19. Am J Physiol Renal Physiol. 2019. PMID: 31215802 Free PMC article.
-
Aging and hemoglobin-induced acute kidney injury.Am J Physiol Renal Physiol. 2013 May 1;304(9):F1167-8. doi: 10.1152/ajprenal.00032.2013. Epub 2013 Jan 30. Am J Physiol Renal Physiol. 2013. PMID: 23364802 Free PMC article. No abstract available.
-
Cytoprotective effects of heme oxygenase in acute renal failure.Contrib Nephrol. 2005;148:70-85. doi: 10.1159/000086044. Contrib Nephrol. 2005. PMID: 15912028 Review.
-
Protective role of heme oxygenase-1 in renal ischemia.Antioxid Redox Signal. 2004 Oct;6(5):867-77. doi: 10.1089/ars.2004.6.867. Antioxid Redox Signal. 2004. PMID: 15345147 Review.
Cited by
-
Oxidative Stress and Cellular Senescence Are Involved in the Aging Kidney.Antioxidants (Basel). 2022 Jan 31;11(2):301. doi: 10.3390/antiox11020301. Antioxidants (Basel). 2022. PMID: 35204184 Free PMC article.
-
Heme Oxygenase-1 in Kidney Health and Disease.Antioxid Redox Signal. 2016 Jul 20;25(3):165-83. doi: 10.1089/ars.2016.6659. Epub 2016 May 26. Antioxid Redox Signal. 2016. PMID: 26906116 Free PMC article. Review.
-
Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial.Clin Kidney J. 2017 Jun;10(3):348-356. doi: 10.1093/ckj/sfw118. Epub 2017 Jan 12. Clin Kidney J. 2017. PMID: 28616213 Free PMC article.
-
Exacerbation of Neonatal Hemolysis and Impaired Renal Iron Handling in Heme Oxygenase 1-Deficient Mice.Int J Mol Sci. 2020 Oct 20;21(20):7754. doi: 10.3390/ijms21207754. Int J Mol Sci. 2020. PMID: 33092142 Free PMC article.
-
Epigenetic profiles of pre-diabetes transitioning to type 2 diabetes and nephropathy.World J Diabetes. 2015 Aug 10;6(9):1113-21. doi: 10.4239/wjd.v6.i9.1113. World J Diabetes. 2015. PMID: 26265998 Free PMC article.
References
-
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000 - PubMed
-
- Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O'Hare AM, Schmader KE, High KP. Acute kidney injury in older adults. J Am Soc Nephrol 22: 28–38, 2011 - PubMed
-
- Arogundade FA, Sanusi AA, Hassan MO, Salawu L, Durosinmi MA, Akinsola A. An appraisal of kidney dysfunction and its risk factors in patients with sickle cell disease. Nephron Clin Pract 118: c225–c231, 2011 - PubMed
-
- Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

