Design and validation of an automated method to detect known adverse drug reactions in MEDLINE: a contribution from the EU-ADR project
- PMID: 23195749
- PMCID: PMC3628051
- DOI: 10.1136/amiajnl-2012-001083
Design and validation of an automated method to detect known adverse drug reactions in MEDLINE: a contribution from the EU-ADR project
Abstract
Objectives: The aim of this research was to automate the search of publications concerning adverse drug reactions (ADR) by defining the queries used to search MEDLINE and by determining the required threshold for the number of extracted publications to confirm the drug/event association in the literature.
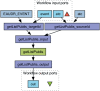
Methods: We defined an approach based on the medical subject headings (MeSH) 'descriptor records' and 'supplementary concept records' thesaurus, using the subheadings 'chemically induced' and 'adverse effects' with the 'pharmacological action' knowledge. An expert-built validation set of true positive and true negative drug/adverse event associations (n=61) was used to validate our method.
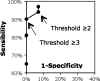
Results: Using a threshold of three of more extracted publications, the automated search method presented a sensitivity of 90% and a specificity of 100%. For nine different drug/event pairs selected, the recall of the automated search ranged from 24% to 64% and the precision from 93% to 48%.
Conclusions: This work presents a method to find previously established relationships between drugs and adverse events in the literature. Using MEDLINE, following a MeSH approach to filter the signals, is a valid option. Our contribution is available as a web service that will be integrated in the final European EU-ADR project (Exploring and Understanding Adverse Drug Reactions by integrative mining of clinical records and biomedical knowledge) automated system.
Figures


References
-
- Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU–ADR Project. Pharmacoepidemiol Drug Saf 2011;20:1–11 - PubMed
-
- Begaud B, Martin K, Haramburu F, et al. Rates of spontaneous reporting of adverse drug reactions in France. JAMA 2002;288:1588. - PubMed
-
- Avillach P, Joubert M, Thiessard F, et al. Design and evaluation of a semantic approach for the homogeneous identification of events in eight patient databases: a contribution to the European EU–ADR project. Stud Health Technol Inform 2010;160:1085–9 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

