A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3
- PMID: 23195960
- PMCID: PMC3554939
- DOI: 10.1074/jbc.M112.425785
A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3
Abstract
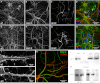
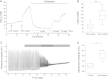
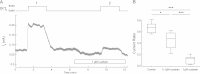
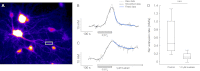
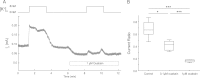
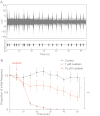
Most neurons co-express two catalytic isoforms of Na,K-ATPase, the ubiquitous α1, and the more selectively expressed α3. Although neurological syndromes are associated with α3 mutations, the specific role of this isoform is not completely understood. Here, we used electrophysiological and Na(+) imaging techniques to study the role of α3 in central nervous system neurons expressing both isoforms. Under basal conditions, selective inhibition of α3 using a low concentration of the cardiac glycoside, ouabain, resulted in a modest increase in intracellular Na(+) concentration ([Na(+)](i)) accompanied by membrane potential depolarization. When neurons were challenged with a large rapid increase in [Na(+)](i), similar to what could be expected following suprathreshold neuronal activity, selective inhibition of α3 almost completely abolished the capacity to restore [Na(+)](i) in soma and dendrite. Recordings of Na,K-ATPase specific current supported the notion that when [Na(+)](i) is elevated in the neuron, α3 is the predominant isoform responsible for rapid extrusion of Na(+). Low concentrations of ouabain were also found to disrupt cortical network oscillations, providing further support for the importance of α3 function in the central nervous system. The α isoforms express a well conserved protein kinase A consensus site, which is structurally associated with an Na(+) binding site. Following activation of protein kinase A, both the α3-dependent current and restoration of dendritic [Na(+)](i) were significantly attenuated, indicating that α3 is a target for phosphorylation and may participate in short term regulation of neuronal function.
Figures

References
-
- Dobretsov M., Stimers J. R. (2005) Neuronal function and α3 isoform of the Na/K-ATPase. Front. Biosci. 10, 2373–2396 - PubMed
-
- Blanco G., Mercer R. W. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–650 - PubMed
-
- Kaplan J. H. (2002) Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

