Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination
- PMID: 23196363
- PMCID: PMC3553911
- DOI: 10.1128/JCM.02417-12
Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination
Abstract
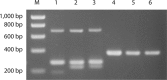
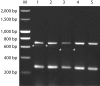
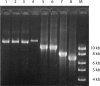
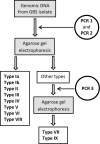
We evaluated three different PCR-based capsular gene typing methods applied to 312 human and bovine Streptococcus agalactiae (group B Streptococcus [GBS]) isolates and compared the results to serotyping results obtained by latex agglutination. Among 281 human isolates 27% could not be typed by latex agglutination. All 312 isolates except 5 could be typed by the three PCR methods combined. Two of these methods were multiplex assays. Among the isolates that were typeable by both latex agglutination and capsular gene typing, 94% showed agreement between the two methods. However, each of the PCR methods showed limitations. One of the methods did not include all 10 recognized serotypes, one misidentified eight isolates of serotypes Ib and IV as serotype Ia, and one did not distinguish between serotypes VII and IX. For five isolates that showed aberrant patterns in the capsular gene typing, long-range PCR targeting the cps operon disclosed large insertions or deletions affecting the cps gene cluster. A sensitive flow cytometric assay based on serotype-specific antibodies applied to 76 selected isolates that were nontypeable by latex agglutination revealed that approximately one-half of these did express capsular polysaccharide. A procedure for convenient and reliable capsular gene typing to be included in epidemiological and surveillance studies of S. agalactiae is proposed.
Figures
References
-
- Dermer P, Lee C, Eggert J, Few B. 2004. A history of neonatal group B streptococcus with its related morbidity and mortality rates in the United States. J. Pediatr. Nurs. 19:357–363 - PubMed
-
- Rodriguez-Granger J, Alvargonzalez JC, Berardi A, Berner R, Kunze M, Hufnagel M, Melin P, Decheva A, Orefici G, Poyart C, Telford J, Efstratiou A, Kilian M, Krizova P, Baldassarri L, Spellerberg B, Puertas A, Rosa-Fraile M. 2012. Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur. J. Clin. Microbiol. Infect. Dis. 31:2097–2104 - PubMed
-
- Sendi P, Johansson L, Nørrby-Teglund A. 2008. Invasive group B streptococcal disease in non-pregnant adults. A review with emphasis on skin and soft-tissue infections. Infection 2:100–111 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

