Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice
- PMID: 23197099
- PMCID: PMC3603337
- DOI: 10.1242/jeb.075598
Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice
Abstract
Small mammals face especially severe thermoregulatory challenges at high altitude because the reduced O2 availability constrains the capacity for aerobic thermogenesis. Adaptive enhancement of thermogenic performance under hypoxic conditions may be achieved via physiological adjustments that occur within the lifetime of individuals (phenotypic plasticity) and/or genetically based changes that occur across generations, but their relative contributions to performance differences between highland and lowland natives are unclear. Here, we examined potentially evolved differences in thermogenic performance between populations of deer mice (Peromyscus maniculatus) that are native to different altitudes. The purpose of the study was to assess the contribution of phenotypic plasticity to population differences in thermogenic performance under hypoxia. We used a common-garden deacclimation experiment to demonstrate that highland deer mice have enhanced thermogenic capacities under hypoxia, and that performance differences between highland and lowland mice persist when individuals are born and reared under common-garden conditions, suggesting that differences in thermogenic capacity have a genetic basis. Conversely, population differences in thermogenic endurance appear to be entirely attributable to physiological plasticity during adulthood. These combined results reveal distinct sources of phenotypic plasticity for different aspects of thermogenic performance, and suggest that thermogenic capacity and endurance may have different mechanistic underpinnings.
Figures
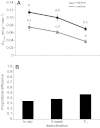
 ) under hypoxic cold stress for highland and lowland deer mice (Peromyscus maniculatus) across the three treatments (in situ, 6 week deacclimation and F1 progeny of deacclimated mice). Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference
) under hypoxic cold stress for highland and lowland deer mice (Peromyscus maniculatus) across the three treatments (in situ, 6 week deacclimation and F1 progeny of deacclimated mice). Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference  in
in  between highland (HA) and lowland (LA) deer mice across treatments.
between highland (HA) and lowland (LA) deer mice across treatments.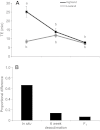
 ) under hypoxic cold stress for highland and lowland deer mice across the three treatments. Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference [1–(TE,LA/TE,HA)] in thermogenic endurance between highland and lowland deer mice across treatments.
) under hypoxic cold stress for highland and lowland deer mice across the three treatments. Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference [1–(TE,LA/TE,HA)] in thermogenic endurance between highland and lowland deer mice across treatments.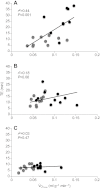
 across the three treatments: (A) in situ, (B) 6 week deacclimation, (C) F1. Black and gray symbols represent highland and lowland deer mice, respectively.
across the three treatments: (A) in situ, (B) 6 week deacclimation, (C) F1. Black and gray symbols represent highland and lowland deer mice, respectively.References
-
- Bangsbo J., Mohr M., Poulsen A., Perez-Gomez J., Krustrup P. (2006). Training and testing the elite athlete. J. Exerc. Sci. Fit. 4, 1-14
-
- Bartholomew G. A. (1968). Body temperature and energy metabolism. In Animal Function Principles and Adaptions (ed. Gordon M. S.). New York, NY: MacMillan;
-
- Bennett A. F. (1991). The evolution of activity capacity. J. Exp. Biol. 160, 1-23 - PubMed
-
- Bjorntorp P. (1991). Importance of fat as a support nutrient for energy: metabolism of athletes. J. Sports Sci. 9 Suppl., 71-76 - PubMed
-
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277-359 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

