Anaesthesia and airway management in mucopolysaccharidosis
- PMID: 23197104
- PMCID: PMC3590422
- DOI: 10.1007/s10545-012-9563-1
Anaesthesia and airway management in mucopolysaccharidosis
Abstract
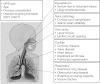
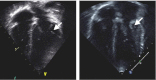
This paper provides a detailed overview and discussion of anaesthesia in patients with mucopolysaccharidosis (MPS), the evaluation of risk factors in these patients and their anaesthetic management, including emergency airway issues. MPS represents a group of rare lysosomal storage disorders associated with an array of clinical manifestations. The high prevalence of airway obstruction and restrictive pulmonary disease in combination with cardiovascular manifestations poses a high anaesthetic risk to these patients. Typical anaesthetic problems include airway obstruction after induction or extubation, intubation difficulties or failure [can't intubate, can't ventilate (CICV)], possible emergency tracheostomy and cardiovascular and cervical spine issues. Because of the high anaesthetic risk, the benefits of a procedure in patients with MPS should always be balanced against the associated risks. Therefore, careful evaluation of anaesthetic risk factors should be made before the procedure, involving evaluation of airways and cardiorespiratory and cervical spine problems. In addition, information on the specific type of MPS, prior history of anaesthesia, presence of cervical instability and range of motion of the temporomandibular joint are important and may be pivotal to prevent complications during anaesthesia. Knowledge of these risk factors allows the anaesthetist to anticipate potential problems that may arise during or after the procedure. Anaesthesia in MPS patients should be preferably done by an experienced (paediatric) anaesthetist, supported by a multidisciplinary team (ear, nose, throat surgeon and intensive care team), with access to all necessary equipment and support.
Conflict of interest statement
Dr Walker, Dr. Belani, Dr. Braunlin, Mr Bruce, Dr. Harmatz, and Dr. Rowe have received a fee for lecturing at an organised education symposium funded by BioMarin Pharmaceutical Inc. Dr. Belani has also participated in a study funded by Biomarin, Inc. and is participating in studies funded by Shire Human Genetic Therapies, Inc. Dr. Jones is a principal investigator on studies funded by BioMarin and received honoraria for speaking and consultancy fees. Dr. Harmatz has provided consulting services to BioMarin Pharmaceutical Inc. and also received research grants, participated in advisory boards, and received speakers honoraria and travel support. Mr. Solanki is a consultant neurosurgeon for the Birmingham Children’s Hospital NHS Foundation Trust and has received speaker’s honorarium and travel support from BioMarin. Dr. Hack and Dr. Valdemarsson have no conflicts of interest
Figures
References
-
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. - DOI - PubMed

