Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error
- PMID: 23197454
- PMCID: PMC3569119
- DOI: 10.1152/jn.00304.2012
Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error
Abstract
We used adaptation to high and low gains in a virtual reality setup of the hand to test competing hypotheses about the excitability changes that accompany motor learning. Excitability was assayed through changes in amplitude of motor evoked potentials (MEPs) in relevant hand muscles elicited with single-pulse transcranial magnetic stimulation (TMS). One hypothesis is that MEPs will either increase or decrease, directly reflecting the effect of low or high gain on motor output. The alternative hypothesis is that MEP changes are not sign dependent but rather serve as a marker of visuomotor learning, independent of performance or visual-to-motor mismatch (i.e., error). Subjects were required to make flexion movements of a virtual forefinger to visual targets. A gain of 1 meant that the excursions of their real finger and virtual finger matched. A gain of 0.25 ("low gain") indicated a 75% reduction in visual versus real finger displacement, a gain of 1.75 ("high gain") the opposite. MEP increases (>40%) were noted in the tonically activated task-relevant agonist muscle for both high- and low-gain perturbations after adaptation reached asymptote with kinematics matched to veridical levels. Conversely, only small changes in excitability occurred in a control task of pseudorandom gains that required adjustments to large errors but in which learning could not accumulate. We conclude that changes in corticospinal excitability are related to learning rather than performance or error.
Figures
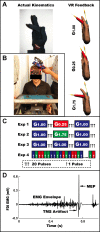
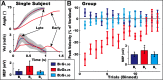


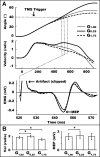
References
-
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol 101: 1776–1782, 2006 - PubMed
-
- Agostino R, Iezzi E, Dinapoli L, Gilio F, Conte A, Mari F, Berardellli A. Effects of 5 Hz subthreshold magnetic stimulation of primary motor cortex on fast finger movements in normal subjects. Exp Brain Res 180: 105–111, 2007 - PubMed
-
- Aranyi Z, Mathis J, Hess CW, Rosler KM. Task-dependent facilitation of motor evoked potentials during dynamic and steady muscle contractions. Muscle Nerve 21: 1309–1316, 1998 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

