Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer
- PMID: 23197490
- PMCID: PMC3514165
- DOI: 10.1093/jnci/djs433
Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer
Abstract
Background: In 2009, the American Society of Clinical Oncology recommended that patients with metastatic colorectal cancer (mCRC) who are candidates for anti-epidermal growth factor receptor (EGFR) therapy have their tumors tested for KRAS mutations because tumors with such mutations do not respond to anti-EGFR therapy. Limiting anti-EGFR therapy to those without KRAS mutations will reserve treatment for those likely to benefit while avoiding unnecessary costs and harm to those who would not. Similarly, tumors with BRAF genetic mutations may not respond to anti-EGFR therapy, though this is less clear. Economic analyses of mutation testing have not fully explored the roles of alternative therapies and resection of metastases.
Methods: This paper is based on a decision analytic framework that forms the basis of a cost-effectiveness analysis of screening for KRAS and BRAF mutations in mCRC in the context of treatment with cetuximab. A cohort of 50 000 patients with mCRC is simulated 10 000 times, with attributes randomly assigned on the basis of distributions from randomized controlled trials.
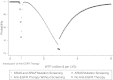
Results: Screening for both KRAS and BRAF mutations compared with the base strategy (of no anti-EGFR therapy) increases expected overall survival by 0.034 years at a cost of $22 033, yielding an incremental cost-effectiveness ratio of approximately $650 000 per additional year of life. Compared with anti-EGFR therapy without screening, adding KRAS testing saves approximately $7500 per patient; adding BRAF testing saves another $1023, with little reduction in expected survival.
Conclusions: Screening for KRAS and BFAF mutation improves the cost-effectiveness of anti-EGFR therapy, but the incremental cost effectiveness ratio remains above the generally accepted threshold for acceptable cost effectiveness ratio of $100 000/quality adjusted life year.
Figures

 : Terminal node (the outcome because of following a path);
: Terminal node (the outcome because of following a path);  : Logical node (logical decisions are made based on logical structure in the node);
: Logical node (logical decisions are made based on logical structure in the node);  : Markov node (indicates the presence of a hidden or shown Markov subtree at the node);
: Markov node (indicates the presence of a hidden or shown Markov subtree at the node);  : Decision node (indicates the point of decision, which in our case is choosing between the four strategies); KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor, VEGF = vascular endothelial growth factor.
: Decision node (indicates the point of decision, which in our case is choosing between the four strategies); KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor, VEGF = vascular endothelial growth factor.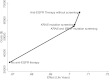


Comment in
-
Gastrointestinal cancer: Screening cost effective, not treatment.Nat Rev Clin Oncol. 2013 Feb;10(2):66. doi: 10.1038/nrclinonc.2012.225. Epub 2012 Dec 11. Nat Rev Clin Oncol. 2013. PMID: 23229185 No abstract available.
-
Re: Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer.J Natl Cancer Inst. 2014 Feb;106(2):djt370. doi: 10.1093/jnci/djt370. Epub 2013 Dec 31. J Natl Cancer Inst. 2014. PMID: 24381069 No abstract available.
References
-
- Winder T Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010; 138(6):2163–2176 - PubMed
-
- Goldberg RM Rothenberg ML Van Cutsem E et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007; 12(1):38–50 - PubMed
-
- Power DG Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010; 28(13):23002309 - PubMed
-
- National Comprehensive Cancer NetworkColon Cancer.. In: NCCN Clinical Practice Guidelines in Oncology.. v.3.2010 ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc; 2011;
-
- Van Cutsem E Nordlinger B Adam R et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006; 42(14):2212–2221 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

