PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice
- PMID: 23197723
- PMCID: PMC6621845
- DOI: 10.1523/JNEUROSCI.1569-12.2012
PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice
Abstract
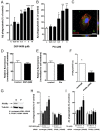
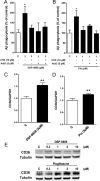
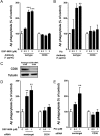
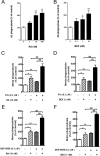
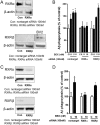
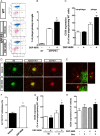
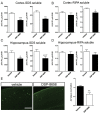
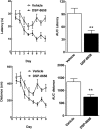
Alzheimer's disease (AD) is characterized by the extracellular deposition of amyloid-β (Aβ), neurofibrillary tangle formation, and a microglial-driven inflammatory response. Chronic inflammatory activation compromises microglial clearance functions. Because peroxisome proliferator-activated receptor γ (PPARγ) agonists suppress inflammatory gene expression, we tested whether activation of PPARγ would also result in improved microglial Aβ phagocytosis. The PPARγ agonist pioglitazone and a novel selective PPARα/γ modulator, DSP-8658, currently in clinical development for the treatment of type 2 diabetes, enhanced the microglial uptake of Aβ in a PPARγ-dependent manner. This PPARγ-stimulated increase of Aβ phagocytosis was mediated by the upregulation of scavenger receptor CD36 expression. In addition, combined treatment with agonists for the heterodimeric binding partners of PPARγ, the retinoid X receptors (RXRs), showed additive enhancement of the Aβ uptake that was mediated by RXRα activation. Evaluation of DSP-8658 in the amyloid precursor protein/presenilin 1 mouse model confirmed an increased microglial Aβ phagocytosis in vivo, which subsequently resulted in a reduction of cortical and hippocampal Aβ levels. Furthermore, DSP-8658-treated mice showed improved spatial memory performance. Therefore, stimulation of microglial clearance by simultaneous activation of the PPARγ/RXRα heterodimer may prove beneficial in prevention of AD.
Figures
Comment in
-
Targeting Alzheimer's pathology through PPARγ signaling: modulation of microglial function.J Neurosci. 2013 Mar 20;33(12):5083-4. doi: 10.1523/JNEUROSCI.0172-13.2013. J Neurosci. 2013. PMID: 23516274 Free PMC article. No abstract available.
References
-
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
