Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation
- PMID: 23197778
- PMCID: PMC3659849
- DOI: 10.5966/sctm.2011-0005
Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation
Abstract
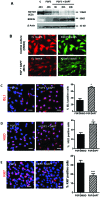
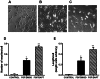
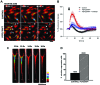
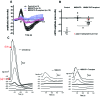
Müller glia with stem cell characteristics have been identified in the adult human eye, and although there is no evidence that they regenerate retina in vivo, they can be induced to grow and differentiate into retinal neurons in vitro. We differentiated human Müller stem cells into retinal ganglion cell (RGC) precursors by stimulation with fibroblast growth factor 2 together with NOTCH inhibition using the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT). Differentiation into RGC precursors was confirmed by gene and protein expression analysis, changes in cytosolic [Ca(2+)] in response to neurotransmitters, and green fluorescent protein (GFP) expression by cells transduced with a transcriptional BRN3b-GFP reporter vector. RGC precursors transplanted onto the inner retinal surface of Lister hooded rats depleted of RGCs by N-methyl-d-aspartate aligned onto the host RGC layer at the site of transplantation but did not extend long processes toward the optic nerve. Cells were observed extending processes into the RGC layer and expressing RGC markers in vivo. This migration was observed only when adjuvant anti-inflammatory and matrix degradation therapy was used for transplantation. RGC precursors induced a significant recovery of RGC function in the transplanted eyes as determined by improvement of the negative scotopic threshold response of the electroretinogram (indicative of RGC function). The results suggest that transplanted RGC precursors may be capable of establishing local interneuron synapses and possibly release neurotrophic factors that facilitate recovery of RGC function. These cells constitute a promising source of cells for cell-based therapies to treat retinal degenerative disease caused by RGC dysfunction.
Figures


References
-
- Randlett O, Norden C, Harris WA. The vertebrate retina: A model for neuronal polarization in vivo. Dev Neurobiol. 2010 - PubMed
-
- Dahlmann-Noor A, Vijay S, Jayaram H, et al. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010;45:333–341. - PubMed
-
- Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

