Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives
- PMID: 23197783
- PMCID: PMC3659848
- DOI: 10.5966/sctm.2011-0036
Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives
Abstract
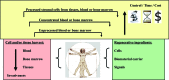
Regenerative therapies in the musculoskeletal system are based on the suitable application of cells, biomaterials, and/or factors. For an effective approach, numerous aspects have to be taken into consideration, including age, disease, target tissue, and several environmental factors. Significant research efforts have been undertaken in the last decade to develop specific cell-based therapies, and in particular adult multipotent mesenchymal stem cells hold great promise for such regenerative strategies. Clinical translation of such therapies, however, remains a work in progress. In the clinical arena, autologous cells have been harvested, processed, and readministered according to protocols distinct for the target application. As outlined in this review, such applications range from simple single-step approaches, such as direct injection of unprocessed or concentrated blood or bone marrow aspirates, to fabrication of engineered constructs by seeding of natural or synthetic scaffolds with cells, which were released from autologous tissues and propagated under good manufacturing practice conditions (for example, autologous chondrocyte implantation). However, only relatively few of these cell-based approaches have entered the clinic, and none of these treatments has become a "standard of care" treatment for an orthopaedic disease to date. The multifaceted reasons for the current status from the medical, research, and regulatory perspectives are discussed here. In summary, this review presents the scientific background, current state, and implications of clinical mesenchymal stem cell application in the musculoskeletal system and provides perspectives for future developments.
Figures
References
-
- Nöth U, Rackwitz L, Steinert AF, et al. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62:765–783. - PubMed
-
- Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–277. - PubMed
-
- Roobrouck VD, Vanuytsel K, Verfaillie CM. Concise review: Culture mediated changes in fate and/or potency of stem cells. Stem Cells. 2011;29:583–589. - PubMed
-
- Jukes JM, van Blitterswijk CA, de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010;4:165–180. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

