Chromosomal duplication is a transient evolutionary solution to stress
- PMID: 23197825
- PMCID: PMC3529009
- DOI: 10.1073/pnas.1211150109
Chromosomal duplication is a transient evolutionary solution to stress
Abstract
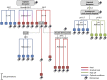
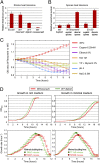
Aneuploidy, an abnormal number of chromosomes, is a widespread phenomenon found in unicellulars such as yeast, as well as in plants and in mammalians, especially in cancer. Aneuploidy is a genome-scale aberration that imposes a severe burden on the cell, yet under stressful conditions specific aneuploidies confer a selective advantage. This dual nature of aneuploidy raises the question of whether it can serve as a stable and sustainable evolutionary adaptation. To clarify this, we conducted a set of laboratory evolution experiments in yeast and followed the long-term dynamics of aneuploidy under diverse conditions. Here we show that chromosomal duplications are first acquired as a crude solution to stress, yet only as transient solutions that are eliminated and replaced by more efficient solutions obtained at the individual gene level. These transient dynamics of aneuploidy were repeatedly observed in our laboratory evolution experiments; chromosomal duplications gained under stress were eliminated not only when the stress was relieved, but even if it persisted. Furthermore, when stress was applied gradually rather than abruptly, alternative solutions appear to have emerged, but not aneuploidy. Our findings indicate that chromosomal duplication is a first evolutionary line of defense, that retains survivability under strong and abrupt selective pressures, yet it merely serves as a "quick fix," whereas more refined and sustainable solutions take over. Thus, in the perspective of genome evolution trajectory, aneuploidy is a useful yet short-lived intermediate that facilitates further adaptation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Torres EM, et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317(5840):916–924. - PubMed
-
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13(3):189–203. - PubMed
-
- Hughes TR, et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat Genet. 2000;25(3):333–337. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

