Folding of large multidomain proteins by partial encapsulation in the chaperonin TRiC/CCT
- PMID: 23197838
- PMCID: PMC3535605
- DOI: 10.1073/pnas.1218836109
Folding of large multidomain proteins by partial encapsulation in the chaperonin TRiC/CCT
Abstract
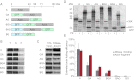
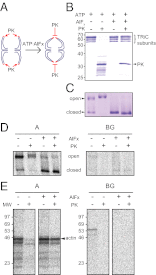
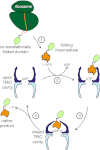
The eukaryotic chaperonin, TRiC/CCT (TRiC, TCP-1 ring complex; CCT, chaperonin containing TCP-1), uses a built-in lid to mediate protein folding in an enclosed central cavity. Recent structural data suggest an effective size limit for the TRiC folding chamber of ∼70 kDa, but numerous chaperonin substrates are substantially larger. Using artificial fusion constructs with actin, an obligate chaperonin substrate, we show that TRiC can mediate folding of large proteins by segmental or domain-wise encapsulation. Single or multiple protein domains up to ∼70 kDa are stably enclosed by stabilizing the ATP-hydrolysis transition state of TRiC. Additional domains, connected by flexible linkers that pass through the central opening of the folding chamber, are excluded and remain accessible to externally added protease. Experiments with the physiological TRiC substrate hSnu114, a 109-kDa multidomain protein, suggest that TRiC has the ability to recognize domain boundaries in partially folded intermediates. In the case of hSnu114, this allows the selective encapsulation of the C-terminal ∼45-kDa domain and segments thereof, presumably reflecting a stepwise folding mechanism. The capacity of the eukaryotic chaperonin to overcome the size limitation of the folding chamber may have facilitated the explosive expansion of the multidomain proteome in eukaryotes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ellis RJ. Protein folding: Importance of the Anfinsen cage. Curr Biol. 2003;13(22):R881–R883. - PubMed
-
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. - PubMed
-
- Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: Physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. - PubMed
-
- Frydman J. Folding of newly translated proteins in vivo: The role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

