Enhanced lithium-induced brain recovery following cranial irradiation is not impeded by inflammation
- PMID: 23197851
- PMCID: PMC3659714
- DOI: 10.5966/sctm.2011-0046
Enhanced lithium-induced brain recovery following cranial irradiation is not impeded by inflammation
Abstract
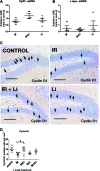
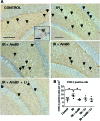
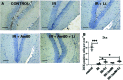
Radiation-induced brain injury occurs in many patients receiving cranial radiation therapy, and these deleterious effects are most profound in younger patients. Impaired neurocognitive functions in both humans and rodents are associated with inflammation, demyelination, and neural stem cell dysfunction. Here we evaluated the utility of lithium and a synthetic retinoid receptor agonist in reducing damage in a model of brain-focused irradiation in juvenile mice. We found that lithium stimulated brain progenitor cell proliferation and differentiation following cranial irradiation while also preventing oligodendrocyte loss in the dentate gyrus of juvenile mice. In response to inflammation induced by radiation, which may have encumbered the optimal reparative action of lithium, we used the anti-inflammatory synthetic retinoid Am80 that is in clinical use in the treatment of acute promyelocytic leukemia. Although Am80 reduced the number of cyclooxygenase-2-positive microglial cells following radiation treatment, it did not enhance lithium-induced neurogenesis recovery, and this alone was not significantly different from the effect of lithium on this proinflammatory response. Similarly, lithium was superior to Am80 in supporting the restoration of new doublecortin-positive neurons following irradiation. These data suggest that lithium is superior in its restorative effects to blocking inflammation alone, at least in the case of Am80. Because lithium has been in routine clinical practice for 60 years, these preclinical studies indicate that this drug might be beneficial in reducing post-therapy late effects in patients receiving cranial radiotherapy and that blocking inflammation in this context may not be as advantageous as previously suggested.
Figures




Similar articles
-
Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation.Radiat Res. 2010 Jan;173(1):49-61. doi: 10.1667/RR1821.1. Radiat Res. 2010. PMID: 20041759 Free PMC article.
-
Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus.Radiat Oncol. 2010 Feb 1;5:6. doi: 10.1186/1748-717X-5-6. Radiat Oncol. 2010. PMID: 20122169 Free PMC article.
-
A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice.J Cereb Blood Flow Metab. 2011 Jan;31(1):222-34. doi: 10.1038/jcbfm.2010.80. Epub 2010 Jun 16. J Cereb Blood Flow Metab. 2011. PMID: 20551971 Free PMC article.
-
Tamibarotene: a candidate retinoid drug for Alzheimer's disease.Biol Pharm Bull. 2012;35(8):1206-12. doi: 10.1248/bpb.b12-00314. Biol Pharm Bull. 2012. PMID: 22863914 Review.
-
Hippocampal dysfunctions caused by cranial irradiation: a review of the experimental evidence.Brain Behav Immun. 2015 Mar;45:287-96. doi: 10.1016/j.bbi.2015.01.007. Epub 2015 Jan 14. Brain Behav Immun. 2015. PMID: 25596174 Review.
Cited by
-
Lithium: A Promising Anticancer Agent.Life (Basel). 2023 Feb 15;13(2):537. doi: 10.3390/life13020537. Life (Basel). 2023. PMID: 36836894 Free PMC article. Review.
-
The crucial role of Atg5 in cortical neurogenesis during early brain development.Sci Rep. 2014 Aug 11;4:6010. doi: 10.1038/srep06010. Sci Rep. 2014. PMID: 25109817 Free PMC article.
-
Effects of Radiation Therapy on Neural Stem Cells.Genes (Basel). 2019 Aug 24;10(9):640. doi: 10.3390/genes10090640. Genes (Basel). 2019. PMID: 31450566 Free PMC article. Review.
-
Glycogen synthase kinase-3 as a therapeutic target for cognitive dysfunction in neuropsychiatric disorders.CNS Drugs. 2015 Jan;29(1):1-15. doi: 10.1007/s40263-014-0213-z. CNS Drugs. 2015. PMID: 25380674 Review.
-
Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro.Oncotarget. 2015 Nov 10;6(35):37083-97. doi: 10.18632/oncotarget.5191. Oncotarget. 2015. PMID: 26397227 Free PMC article.
References
-
- Hertzberg H, Huk WJ, Ueberall MA, et al. CNS late effects after ALL therapy in childhood. Part I: Neuroradiological findings in long-term survivors of childhood ALL—An evaluation of the interferences between morphology and neuropsychological performance. The German Late Effects Working Group. Med Pediatr Oncol. 1997;28:387–400. - PubMed
-
- Riva D, Giorgi C. The neurodevelopmental price of survival in children with malignant brain tumours. Childs Nerv Syst. 2000;16:751–754. - PubMed
-
- Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

