Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling
- PMID: 23197854
- PMCID: PMC3659711
- DOI: 10.5966/sctm.2012-0005
Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling
Abstract
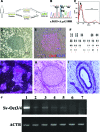
We investigated retinitis pigmentosa (RP) caused by a mutation in the gene rhodopsin (RHO) with a patient-specific rod cell model generated from induced pluripotent stem cells (iPSCs) derived from an RP patient. To generate the iPSCs and to avoid the unpredictable side effects associated with retrovirus integration at random loci in the host genome, a nonintegrating Sendai-virus vector was installed with four key reprogramming gene factors (POU5F1, SOX2, KLF4, and c-MYC) in skin cells from an RP patient. Subsequent selection of the iPSC lines was on the basis of karyotype analysis as well as in vitro and in vivo pluripotency tests. Using a serum-free, chemically defined, and stepwise differentiation method, the expressions of specific markers were sequentially induced in a neural retinal progenitor, a retinal pigment epithelial (RPE) progenitor, a photoreceptor precursor, RPE cells, and photoreceptor cells. In the differentiated rod cells, diffused distribution of RHO protein in cytoplasm and expressions of endoplasmic reticulum (ER) stress markers strongly indicated the involvement of ER stress. Furthermore, the rod cell numbers decreased significantly after successive culture, suggesting an in vitro model of rod degeneration. Thus, from integration-free patient-specific iPSCs, RP patient-specific rod cells were generated in vitro that recapitulated the disease feature and revealed evidence of ER stress in this patient, demonstrating its utility for disease modeling in vitro.
Figures




References
-
- Boucherie C, Sowden JC, Ali RR. Induced pluripotent stem cell technology for generating photoreceptors. Regen Med. 2011;6:469–479. - PubMed
-
- Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457–466. - PubMed
-
- Osakada F, Hirami Y, Takahashi M. Stem cell biology and cell transplantation therapy in the retina. Biotechnol Genet Eng Rev. 2010;26:297–334. - PubMed
-
- Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

