Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering
- PMID: 23197855
- PMCID: PMC3659717
- DOI: 10.5966/sctm.2012-0002
Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering
Abstract
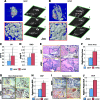
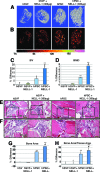
Adipose tissue is an ideal source of mesenchymal stem cells for bone tissue engineering: it is largely dispensable and readily accessible with minimal morbidity. However, the stromal vascular fraction (SVF) of adipose tissue is a heterogeneous cell population, which leads to unreliable bone formation. In the present study, we prospectively purified human perivascular stem cells (PSCs) from adipose tissue and compared their bone-forming capacity with that of traditionally derived SVF. PSCs are a population (sorted by fluorescence-activated cell sorting) of pericytes (CD146+CD34-CD45-) and adventitial cells (CD146-CD34+CD45-), each of which we have previously reported to have properties of mesenchymal stem cells. Here, we found that PSCs underwent osteogenic differentiation in vitro and formed bone after intramuscular implantation without the need for predifferentiation. We next sought to optimize PSCs for in vivo bone formation, adopting a demineralized bone matrix for osteoinduction and tricalcium phosphate particle formulation for protein release. Patient-matched, purified PSCs formed significantly more bone in comparison with traditionally derived SVF by all parameters. Recombinant bone morphogenetic protein 2 increased in vivo bone formation but with a massive adipogenic response. In contrast, recombinant Nel-like molecule 1 (NELL-1; a novel osteoinductive growth factor) selectively enhanced bone formation. These studies suggest that adipose-derived human PSCs are a new cell source for future efforts in skeletal regenerative medicine. Moreover, PSCs are a stem cell-based therapeutic that is readily approvable by the U.S. Food and Drug Administration, with potentially increased safety, purity, identity, potency, and efficacy. Finally, NELL-1 is a candidate growth factor able to induce human PSC osteogenesis.
Figures




References
-
- Laurencin C, Khan Y. Bone Graft Substitute Materials. emedicine 2004. [Accessed February 16, 2012]. Available at http://emedicine.medscape.com/article/1230616-overview.
-
- Laurie SW, Kaban LB, Mulliken JB, et al. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933–938. - PubMed
-
- Frodel JL, Jr., Marentette LJ, Quatela VC, et al. Calvarial bone graft harvest. Techniques, considerations, and morbidity. Arch Otolaryngol Head Neck Surg. 1993;119:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

