Targeting p38 mitogen-activated protein kinase signaling restores subventricular zone neural stem cells and corrects neuromotor deficits in Atm knockout mouse
- PMID: 23197859
- PMCID: PMC3659722
- DOI: 10.5966/sctm.2011-0063
Targeting p38 mitogen-activated protein kinase signaling restores subventricular zone neural stem cells and corrects neuromotor deficits in Atm knockout mouse
Abstract
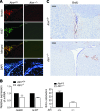
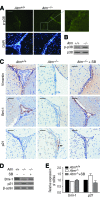
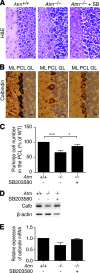
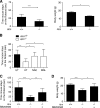
Ataxia-telangiectasia (A-T) is a progressive degenerative disorder that results in major neurological disability. In A-T patients, necropsy has revealed atrophy of cerebellar cortical layers along with Purkinje and granular cell loss. We have previously identified an oxidative stress-mediated increase in phospho-p38 mitogen-activated protein kinase (MAPK) and the resultant downregulation of Bmi-1 and upregulation of p21 as key components of the mechanism causing defective proliferation of neural stem cells (NSCs) isolated from the subventricular zone (SVZ) of Atm(-/-) mice. However, the in vivo aspect of alteration in SVZ tissue and the functional significance of p38MAPK activation in NSCs for neuropathogenesis of ATM deficiency remain unknown. Here we show that the NSC population was abnormally decreased in the SVZ of 3-month-old Atm(-/-) mice; this decrease was accompanied by p38MAPK activation. However, after a 2-month treatment with the p38MAPK inhibitor SB203580, starting at 1 month old, Atm(-/-) mice showed restoration of normal levels of Bmi-1 and p21 with the rescue of NSC population in the SVZ. In addition, treated Atm(-/-) mice exhibited more Purkinje cells in the cerebellum. Most importantly, motor coordination of Atm(-/-) mice was significantly improved in the treatment group. Our results show for the first time in vivo evidence of depleted NSCs in the SVZ of Atm(-/-) mice and also demonstrate that pharmacologic inhibition of p38MAPK signaling has the potential to treat neurological defects of A-T. This study provides a promising approach targeting the oxidative stress-dependent p38 signaling pathway not only for A-T but also for other neurodegenerative disorders.
Figures
Similar articles
-
Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling.Stem Cells. 2009 Aug;27(8):1987-98. doi: 10.1002/stem.125. Stem Cells. 2009. PMID: 19544430
-
p38 MAPK-Mediated Bmi-1 down-regulation and defective proliferation in ATM-deficient neural stem cells can be restored by Akt activation.PLoS One. 2011 Jan 28;6(1):e16615. doi: 10.1371/journal.pone.0016615. PLoS One. 2011. PMID: 21305053 Free PMC article.
-
Activation of AMP-activated protein kinase in cerebella of Atm-/- mice is attributable to accumulation of reactive oxygen species.Biochem Biophys Res Commun. 2012 Feb 10;418(2):267-72. doi: 10.1016/j.bbrc.2012.01.008. Epub 2012 Jan 10. Biochem Biophys Res Commun. 2012. PMID: 22260947 Free PMC article.
-
ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia.Int J Radiat Biol. 1999 Oct;75(10):1201-14. doi: 10.1080/095530099139359. Int J Radiat Biol. 1999. PMID: 10549596 Review.
-
ATM and the molecular pathogenesis of ataxia telangiectasia.Annu Rev Pathol. 2012;7:303-21. doi: 10.1146/annurev-pathol-011811-132509. Epub 2011 Oct 24. Annu Rev Pathol. 2012. PMID: 22035194 Review.
Cited by
-
Increased gene dosage of Ink4/Arf and p53 delays age-associated central nervous system functional decline.Aging Cell. 2015 Aug;14(4):710-4. doi: 10.1111/acel.12343. Epub 2015 May 20. Aging Cell. 2015. PMID: 25990896 Free PMC article.
-
Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro- and systemic inflammation in a model of Gulf War Illness.Redox Biol. 2020 Jan;28:101389. doi: 10.1016/j.redox.2019.101389. Epub 2019 Nov 18. Redox Biol. 2020. PMID: 31778892 Free PMC article.
-
GSE4 peptide suppresses oxidative and telomere deficiencies in ataxia telangiectasia patient cells.Cell Death Differ. 2019 Oct;26(10):1998-2014. doi: 10.1038/s41418-018-0272-7. Epub 2019 Jan 22. Cell Death Differ. 2019. PMID: 30670828 Free PMC article.
-
Inhibition of astrocyte FAK-JNK signaling promotes subventricular zone neurogenesis through CNTF.Glia. 2018 Nov;66(11):2456-2469. doi: 10.1002/glia.23498. Glia. 2018. PMID: 30500112 Free PMC article.
-
Pathophysiological basis and promise of experimental therapies for Gulf War Illness, a chronic neuropsychiatric syndrome in veterans.Psychopharmacology (Berl). 2023 Apr;240(4):673-697. doi: 10.1007/s00213-023-06319-5. Epub 2023 Feb 15. Psychopharmacology (Berl). 2023. PMID: 36790443 Review.
References
-
- Gatti RA, Boder E, Vinters HV, et al. Ataxia-telangiectasia: An interdisciplinary approach to pathogenesis. Medicine (Baltimore) 1991;70:99–117. - PubMed
-
- Wong PKY, Kim J, Kim SJ, et al. Oxidative stress-mediated neurodegeneration: A tale of two models. In: McNeil AS, editor. Neurodegeneration: Theory, Disorders and Treatments. Nova Science Publishers, Inc; 2010. pp. 1–44.
-
- Paula-Barbosa MM, Ruela C, Tavares MA, et al. Cerebellar cortex ultrastructure in ataxia-telangiectasia. Ann Neurol. 1983;13:297–302. - PubMed
-
- Bottini AR, Gatti RA, Wirenfeldt M, et al. Heterotopic Purkinje cells in ataxia-telangiectasia. Neuropathology. 2012;32:23–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

