Serum- and stromal cell-free hypoxic generation of embryonic stem cell-derived hematopoietic cells in vitro, capable of multilineage repopulation of immunocompetent mice
- PMID: 23197864
- PMCID: PMC3659726
- DOI: 10.5966/sctm.2012-0020
Serum- and stromal cell-free hypoxic generation of embryonic stem cell-derived hematopoietic cells in vitro, capable of multilineage repopulation of immunocompetent mice
Abstract
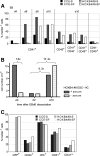
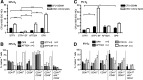
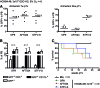
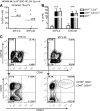
Induced pluripotent stem cells (iPSCs) may become a promising source for the generation of patient-specific hematopoietic stem cells (HSCs) in vitro. A crucial prerequisite will be the availability of reliable protocols for the directed and efficient differentiation toward HSCs. So far, the most robust strategy for generating HSCs from pluripotent cells in vitro has been established in the mouse model involving ectopic expression of the human transcription factor HOXB4. However, most differentiation protocols include coculture on a xenogenic stroma cell line and the use of animal serum. Involvement of any of both would pose a major barrier to the translation of those protocols to human autologous iPSCs intended for clinical use. Therefore, we asked whether long-term repopulating HSCs can, in principle, be generated from embryonic stem cells without stroma cells or serum. Here, we showed that long-term multilineage engraftment could be accomplished in immunocompetent mice when HSCs were generated in serum-free medium without stroma cell support and when hypoxic conditions were used. Under those conditions, HOXB4(+) embryonic stem cell-derived hematopoietic stem and progenitor cells were immunophenotypically similar to definitive bone marrow resident E-SLAM(+) (CD150(+)CD48(-)CD45(+)CD201(+)) HSCs. Thus, our findings may ease the development of definitive, adult-type HSCs from pluripotent stem cells, entirely in vitro.
Figures


References
-
- Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. - PubMed
-
- Rideout WM, 3rd, Hochedlinger K, Kyba M, et al. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. - PubMed
-
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: Advances and questions. Development. 2011;138:1017–1031. - PubMed
-
- Medvinsky A, Dziercak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

