An abundant perivascular source of stem cells for bone tissue engineering
- PMID: 23197874
- PMCID: PMC3659737
- DOI: 10.5966/sctm.2012-0053
An abundant perivascular source of stem cells for bone tissue engineering
Abstract
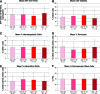
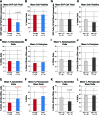
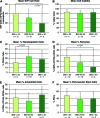
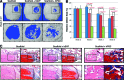
Adipose tissue is an ideal mesenchymal stem cell (MSC) source, as it is dispensable and accessible with minimal morbidity. However, the stromal vascular fraction (SVF) of adipose tissue is a heterogeneous cell population, which has disadvantages for tissue regeneration. In the present study, we prospectively purified human perivascular stem cells (PSCs) from n = 60 samples of human lipoaspirate and documented their frequency, viability, and variation with patient demographics. PSCs are a fluorescence-activated cell sorting-sorted population composed of pericytes (CD45-, CD146+, CD34-) and adventitial cells (CD45-, CD146-, CD34+), each of which we have previously reported to have properties of MSCs. Here, we found that PSCs make up, on average, 43.2% of SVF from human lipoaspirate (19.5% pericytes and 23.8% adventitial cells). These numbers were minimally changed by age, gender, or body mass index of the patient or by length of refrigerated storage time between liposuction and processing. In a previous publication, we observed that human PSCs (hPSCs) formed significantly more bone in vivo in comparison with unsorted human SVF (hSVF) in an intramuscular implantation model. We now extend this finding to a bone injury model, observing that purified hPSCs led to significantly greater healing of mouse critical-size calvarial defects than hSVF (60.9% healing as opposed to 15.4% healing at 2 weeks postoperative by microcomputed tomography analysis). These studies suggest that adipose-derived hPSCs are a new cell source for future efforts in skeletal regenerative medicine. Moreover, hPSCs are a stem cell-based therapeutic that is readily approvable by the U.S. Food and Drug Administration, with potentially increased safety, purity, identity, potency, and efficacy.
Figures



References
-
- Cui L, Liu B, Liu G, et al. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 2007;28:5477–5486. - PubMed
-
- Tapp H, Hanley EN, Jr., Patt JC, et al. Adipose-derived stem cells: Characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 2009;234:1–9. - PubMed
-
- Safwani WK, Makpol S, Sathapan S, et al. The impact of long-term in vitro expansion on the senescence-associated markers of human adipose-derived stem cells. Appl Biochem Biotechnol. 2012;166:2101–2113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

