Concentration-driven growth of model protocell membranes
- PMID: 23198690
- PMCID: PMC3530389
- DOI: 10.1021/ja310382d
Concentration-driven growth of model protocell membranes
Abstract
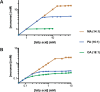
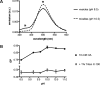
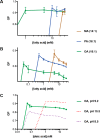
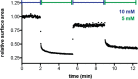
The first protocell membranes may have assembled from fatty acids and related single-chain lipids available in the prebiotic environment. Prior to the evolution of complex cellular machinery, spontaneous protocell membrane growth and division had to result from the intrinsic physicochemical properties of these molecules, in the context of specific environmental conditions. Depending on the nature of the chemical and physical environment, fatty acids can partition between several different phases, including soluble monomers, micelles, and lamellar vesicles. Here we address the concentration dependence of fatty acid aggregation, which is dominated by entropic considerations. We quantitatively distinguish between fatty acid phases using a combination of physical and spectroscopic techniques, including the use of the fluorescent fatty acid analogue Laurdan, whose emission spectrum is sensitive to structural differences between micellar and lamellar aggregates. We find that the monomer-aggregate transition largely follows a characteristic pseudophase model of molecular aggregation but that the composition of the aggregate phase is also concentration dependent. At low amphiphile concentrations above the critical aggregate concentration, vesicles coexist with a significant proportion of micelles, while more concentrated solutions favor the lamellar vesicle phase. We subsequently show that the micelle-vesicle equilibrium can be used to drive the growth of pre-existing vesicles upon an increase in amphiphile concentration either through solvent evaporation or following the addition of excess lipids. We propose a simple model for a primitive environmentally driven cell cycle, in which protocell membrane growth results from evaporative concentration, followed by shear force or photochemically induced division.
Figures




References
-
- Gebicki J. M.; Hicks M. Nature 1973, 243, 232–234. - PubMed
-
- Hargreaves W. R.; Deamer D. W. Biochemistry 1978, 17, 3759–3768. - PubMed
-
- Rushdi A. I.; Simoneit B. R. Origins Life Evol. Biospheres 2001, 31, 103–118. - PubMed
-
- Simoneit B. R. T. Adv. Space Res. 2004, 33, 88–94.
-
- Yuen G. U.; Kvenvolden K. A. Nature 1973, 246, 301–303.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

