Evaluation of the hematoma consequences, neurobehavioral profiles, and histopathology in a rat model of pontine hemorrhage
- PMID: 23198805
- PMCID: PMC3569075
- DOI: 10.3171/2012.10.JNS111836
Evaluation of the hematoma consequences, neurobehavioral profiles, and histopathology in a rat model of pontine hemorrhage
Abstract
Object: Primary pontine hemorrhage (PPH) represents approximately 7% of all intracerebral hemorrhages (ICHs) and is a clinical condition of which little is known. The aim of this study was to characterize the early brain injury, neurobehavioral outcome, and long-term histopathology in a novel preclinical rat model of PPH.
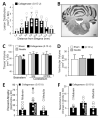
Methods: The authors stereotactically infused collagenase (Type VII) into the ventral pontine tegmentum of the rats, in accordance with the most commonly affected clinical region. Measures of cerebrovascular permeability (brain water content, hemoglobin assay, Evans blue, collagen Type IV, ZO-1, and MMP-2 and MMP-9) and neurological deficit were quantified at 24 hours postinfusion (Experiment 1). Functional outcome was measured over a 30-day period using a vertebrobasilar scale (the modified Voetsch score), open field, wire suspension, beam balance, and inclined-plane tests (Experiment 2). Neurocognitive ability was determined at Week 3 using the rotarod (motor learning), T-maze (working memory), and water maze (spatial learning and memory) (Experiment 3), followed by histopathological analysis 1 week later (Experiment 4).
Results: Stereotactic collagenase infusion caused dose-dependent elevations in hematoma volume, brain edema, neurological deficit, and blood-brain barrier rupture, while physiological variables remained stable. Functional outcomes mostly normalized by Week 3, whereas neurocognitive deficits paralleled the cystic cavitary lesion at 30 days. Obstructive hydrocephalus did not develop despite a clinically relevant 30-day mortality rate (approximately 54%).
Conclusions: These results suggest that the model can mimic several translational aspects of pontine hemorrhage in humans and can be used in the evaluation of potential preclinical therapeutic interventions.
Figures


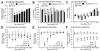

Comment in
-
Letter to the Editor: pontine hemorrhage.J Neurosurg. 2013 May;118(5):1152. doi: 10.3171/2012.12.JNS122420. Epub 2013 Mar 22. J Neurosurg. 2013. PMID: 23521553 No abstract available.
-
Response.J Neurosurg. 2013 May;118(5):1152-3. J Neurosurg. 2013. PMID: 23776937 No abstract available.
References
-
- Andaluz N, Zuccarello M, Wagner KR. Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:385–393. - PubMed
-
- Avendaño C, Machín R, Bermejo PE, Lagares A. Neuron numbers in the sensory trigeminal nuclei of the rat: A GABA- and glycine-immunocytochemical and stereological analysis. J Comp Neurol. 2005;493:538–553. - PubMed
-
- Balci K, Asil T, Kerimoglu M, Celik Y, Utku U. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg. 2005;108:36–39. - PubMed
-
- Batton RR, III, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174:281–305. - PubMed
-
- Bermejo PE, Jiménez CE, Torres CV, Avendaño C. Quantitative stereological evaluation of the gracile and cuneate nuclei and their projection neurons in the rat. J Comp Neurol. 2003;463:419–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

