Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation
- PMID: 23198981
- PMCID: PMC3526410
- DOI: 10.1186/1742-2094-9-262
Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation
Abstract
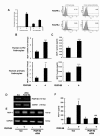
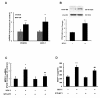
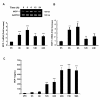
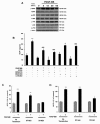
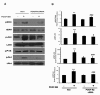
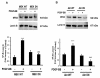
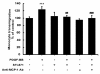
Chemokine (C-C motif) ligand 2, also known as monocyte chemoattractant protein 1 (MCP-1) is an important factor for the pathogenesis of HIV-associated neurocognitive disorders (HAND). The mechanisms of MCP-1-mediated neuropathogenesis, in part, revolve around its neuroinflammatory role and the recruitment of monocytes into the central nervous system (CNS) via the disrupted blood-brain barrier (BBB). We have previously demonstrated that HIV-1/HIV-1 Tat upregulate platelet-derived growth factor (PDGF)-BB, a known cerebrovascular permeant; subsequently, the present study was aimed at exploring the regulation of MCP-1 by PDGF-BB in astrocytes with implications in HAND. Specifically, the data herein demonstrate that exposure of human astrocytes to HIV-1 LAI elevated PDGF-B and MCP-1 levels. Furthermore, treating astrocytes with the human recombinant PDGF-BB protein significantly increased the production and release of MCP-1 at both the RNA and protein levels. MCP-1 induction was regulated by activation of extracellular-signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinases and phosphatidylinositol 3-kinase (PI3K)/Akt pathways and the downstream transcription factor, nuclear factor κB (NFκB). Chromatin immunoprecipitation (ChIP) assays demonstrated increased binding of NFκB to the human MCP-1 promoter following PDGF-BB exposure. Conditioned media from PDGF-BB-treated astrocytes increased monocyte transmigration through human brain microvascular endothelial cells (HBMECs), an effect that was blocked by STI-571, a tyrosine kinase inhibitor (PDGF receptor (PDGF-R) blocker). PDGF-BB-mediated release of MCP-1 was critical for increased permeability in an in vitro BBB model as evidenced by blocking antibody assays. Since MCP-1 is linked to disease severity, understanding its modulation by PDGF-BB could aid in understanding the proinflammatory responses in HAND. These results suggest that astrocyte activation by PDGF-BB exaggerates monocyte recruitment into the brain via MCP-1 and underscores the critical role astrocytes play in HAND.
Figures

References
-
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
-
- Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood–brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–2959. - PubMed
-
- Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. - PubMed
-
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

