Lysosome-targeted octadecyl-rhodamine B-liposomes enhance lysosomal accumulation of glucocerebrosidase in Gaucher's cells in vitro
- PMID: 23199221
- PMCID: PMC3644353
- DOI: 10.2217/nnm.12.138
Lysosome-targeted octadecyl-rhodamine B-liposomes enhance lysosomal accumulation of glucocerebrosidase in Gaucher's cells in vitro
Abstract
Aim: We hypothesized that liposomes modified with lysosomotropic octadecyl-rhodamine B (Rh) and loaded with therapeutic glucocerebroside velaglucerase alfa (VPRIV™) will improve lysosomal delivery of the enzyme into Gaucher's cells.
Materials & methods: Confocal microscopy and flow cytometry were used to evaluate the ability of Rh-modified liposomes loaded with VPRIV to improve the lysosomal targeting in monocyte-derived macrophages and Gaucher's fibroblasts.
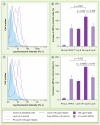
Results: Confocal microscopy demonstrated that Rh-modified liposomes localized primarily in the lysosomes. As confirmed by flow cytometry using specific substrate 5-(pentafluorobenzoylamino)fluorescein diglucoside, intralysosomal accumulation of VPRIV in the cells treated with Rh-modified liposomes was significantly increased (up to 68%) relative to the cells treated with plain liposomes or free VPRIV.
Conclusion: Rh-modified lysosomotropic liposomes can improve lysosomal accumulation of liposomal enzymes both in nonphagocytic Gaucher's fibroblasts and phagocytic monocyte-derived macrophages.
Figures




References
-
- Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. - PubMed
-
- Van Der Ploeg AT, Reuser AJ. Pompe’s disease. Lancet. 2008;372(9646):1342–1353. - PubMed
-
- Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372(9647):1427–1435. - PubMed
-
- Grabowski GA, Hopkin RJ. Enzyme therapy for lysosomal storage disease: principles, practice, and prospects. Ann. Rev. Genomics Hum. Genet. 2003;4:403–436. - PubMed
-
- Jmoudiak M, Futerman AH. Gaucher disease: pathological mechanisms and modern management. Br. J. Haematol. 2005;129(2):178–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
