Assessing the metabolic effects of prednisolone in healthy volunteers using urine metabolic profiling
- PMID: 23199229
- PMCID: PMC4064315
- DOI: 10.1186/gm395
Assessing the metabolic effects of prednisolone in healthy volunteers using urine metabolic profiling
Abstract
Background: Glucocorticoids, such as prednisolone, are widely used anti-inflammatory drugs, but therapy is hampered by a broad range of metabolic side effects including skeletal muscle wasting and insulin resistance. Therefore, development of improved synthetic glucocorticoids that display similar efficacy as prednisolone but reduced side effects is an active research area. For efficient development of such new drugs, in vivo biomarkers, which can predict glucocorticoid metabolic side effects in an early stage, are needed. In this study, we aim to provide the first description of the metabolic perturbations induced by acute and therapeutic treatments with prednisolone in humans using urine metabolomics, and to derive potential biomarkers for prednisolone-induced metabolic effects.
Methods: A randomized, double blind, placebo-controlled trial consisting of two protocols was conducted in healthy men. In protocol 1, volunteers received placebo (n = 11) or prednisolone (7.5 mg (n = 11), 15 mg (n = 13) or 30 mg (n = 12)) orally once daily for 15 days. In protocol 2, volunteers (n = 6) received placebo at day 0 and 75 mg prednisolone at day 1. We collected 24 h urine and serum samples at baseline (day 0), after a single dose (day 1) and after prolonged treatment (day 15) and obtained mass-spectrometry-based urine and serum metabolic profiles.
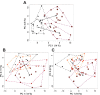
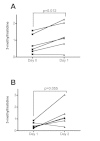
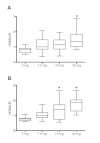
Results: At day 1, high-dose prednisolone treatment increased levels of 13 and 10 proteinogenic amino acids in urine and serum respectively, as well as levels of 3-methylhistidine, providing evidence for an early manifestation of glucocorticoid-induced muscle wasting. Prednisolone treatment also strongly increased urinary carnitine derivatives at day 1 but not at day 15, which might reflect adaptive mechanisms under prolonged treatment. Finally, urinary levels of proteinogenic amino acids at day 1 and of N-methylnicotinamide at day 15 significantly correlated with the homeostatic model assessment of insulin resistance and might represent biomarkers for prednisolone-induced insulin resistance.
Conclusion: This study provides evidence that urinary metabolomics represents a noninvasive way of monitoring the effect of glucocorticoids on muscle protein catabolism after a single dose and can derive new biomarkers of glucocorticoid-induced insulin resistance. It might, therefore, help the development of improved synthetic glucocorticoids.
Trial registration: ClinicalTrials.gov NCT00971724.
Keywords: 3-methylhistidine; HOMA-IR; aminoaciduria; metabolomics; prednisolone; urine.
Figures
References
-
- Jacobson PB, von Geldern TW, Ohman L, Osterland M, Wang J, Zinker B, Wilcox D, Nguyen PT, Mika A, Fung S. et al.Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. The Journal of pharmacology and experimental therapeutics. 2005;4:191–200. doi: 10.1124/jpet.104.081257. - DOI - PubMed
-
- Schacke H, Schottelius A, Docke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA. 2004;4:227–232. doi: 10.1073/pnas.0300372101. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

