Modeling of wildlife-associated zoonoses: applications and caveats
- PMID: 23199265
- PMCID: PMC3525896
- DOI: 10.1089/vbz.2012.0987
Modeling of wildlife-associated zoonoses: applications and caveats
Abstract
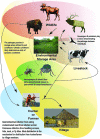
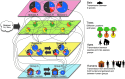
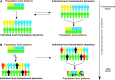
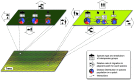
Wildlife species are identified as an important source of emerging zoonotic disease. Accordingly, public health programs have attempted to expand in scope to include a greater focus on wildlife and its role in zoonotic disease outbreaks. Zoonotic disease transmission dynamics involving wildlife are complex and nonlinear, presenting a number of challenges. First, empirical characterization of wildlife host species and pathogen systems are often lacking, and insight into one system may have little application to another involving the same host species and pathogen. Pathogen transmission characterization is difficult due to the changing nature of population size and density associated with wildlife hosts. Infectious disease itself may influence wildlife population demographics through compensatory responses that may evolve, such as decreased age to reproduction. Furthermore, wildlife reservoir dynamics can be complex, involving various host species and populations that may vary in their contribution to pathogen transmission and persistence over space and time. Mathematical models can provide an important tool to engage these complex systems, and there is an urgent need for increased computational focus on the coupled dynamics that underlie pathogen spillover at the human-wildlife interface. Often, however, scientists conducting empirical studies on emerging zoonotic disease do not have the necessary skill base to choose, develop, and apply models to evaluate these complex systems. How do modeling frameworks differ and what considerations are important when applying modeling tools to the study of zoonotic disease? Using zoonotic disease examples, we provide an overview of several common approaches and general considerations important in the modeling of wildlife-associated zoonoses.
Figures


References
-
- Adjemian J. Girvetz E. Beckett L, et al. Analysis of Genetic Algorithm for Rule-Set Production (GARP) modeling approach for predicting distributions of fleas implicated as vectors of plague, Yersinia pestis, in California. J Med Entomol. 2006;43:93–103. - PubMed
-
- Alexander KA. McNutt JW. Human behavior influences infectious disease emergence at the human-animal interface. Front Ecol Environ. 2010
-
- Anderson R. May RM. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford; 1991. p. 757.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

