Retinitis pigmentosa and ocular blood flow
- PMID: 23199279
- PMCID: PMC3531263
- DOI: 10.1186/1878-5085-3-17
Retinitis pigmentosa and ocular blood flow
Abstract
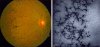
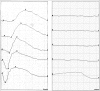
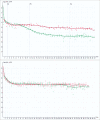
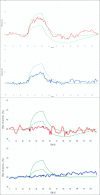
Is the concept of integrative, preventive and personalised medicine applicable to the relationship between retinitis pigmentosa (RP) and ocular blood flow (OBF)? RP encompasses a group of hereditary diseases of the posterior segment of the eye characterised by degeneration, atrophy and finally loss of photoreceptors and retinal pigment epithelium, leading to progressive visual loss. Many different mutations affecting different genes can lead to the clinical picture of RP. Even though the disease has a clear genetic background, there are obviously other factors influencing the manifestation and progression of RP. In this review, we focus on the role of OBF. There is evidence that, in PR patients, OBF is more reduced than one would expect secondary to the retinal atrophy. The main cause of this additional component seems to be primary vascular dysregulation (PVD) syndrome. As PVD syndrome is partly treatable, a vascular evaluation of RP patients is meaningful. Based on the outcome, a targeted individualised, preventive or supportive treatment might be introduced in selected RP patients.
Figures









References
-
- Grunwald JE, Maguire AM, Dupont J. Retinal hemodynamics in retinitis pigmentosa. Am J Ophthalmol. 1996;122:502–508. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

