Locating a lipid at the portal to the lipoxygenase active site
- PMID: 23200047
- PMCID: PMC3512035
- DOI: 10.1016/j.bpj.2012.10.002
Locating a lipid at the portal to the lipoxygenase active site
Abstract
Lipoxygenase enzymes initiate diverse signaling pathways by specifically directing oxygen to different carbons of arachidonate and other polyunsaturated acyl chains, but structural origins of this specificity have remained unclear. We therefore determined the nature of the lipoxygenase interaction with the polar-end of a paramagnetic lipid by electron paramagnetic resonance spectroscopy. Distances between selected grid points on soybean seed lipoxygenase-1 (SBL1) and a lysolecithin spin-labeled on choline were measured by pulsed (electron) dipolar spectroscopy. The protein grid was designed by structure-based modeling so that five natural side chains were replaced with spin labels. Pairwise distances in 10 doubly spin-labeled mutants were examined by pulsed dipolar spectroscopy, and a fit to the model was optimized. Finally, experimental distances between the lysolecithin spin and each single spin site on SBL1 were also obtained. With these 15 distances, distance geometry localized the polar-end and the spin of the lysolecithin to the region between the two domains in the SBL1 structure, nearest to E236, K260, Q264, and Q544. Mutation of a nearby residue, E256A, relieved the high pH requirement for enzyme activity of SBL1 and allowed lipid binding at pH 7.2. This general approach could be used to locate other flexible molecules in macromolecular complexes.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

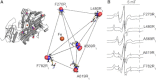
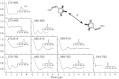
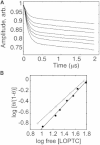
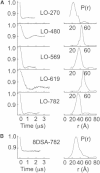
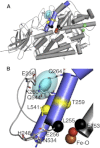
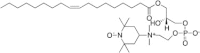
Comment in
-
Taking the pulse of protein interactions by EPR spectroscopy.Biophys J. 2012 Nov 21;103(10):2047-8. doi: 10.1016/j.bpj.2012.10.005. Epub 2012 Nov 20. Biophys J. 2012. PMID: 23200037 Free PMC article.
References
-
- Rådmark O., Werz O., Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 2007;32:332–341. - PubMed
-
- Boyington J.C., Gaffney B.J., Amzel L.M. The three-dimensional structure of an arachidonic acid 15-lipoxygenase. Science. 1993;260:1482–1486. - PubMed
-
- Minor W., Steczko J., Axelrod B. Crystal structure of soybean lipoxygenase L-1 at 1.4 Å resolution. Biochemistry. 1996;35:10687–10701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

