Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of α-synuclein oligomers and decrease of neurites
- PMID: 23200460
- PMCID: PMC3570625
- DOI: 10.1016/j.neurobiolaging.2012.11.001
Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of α-synuclein oligomers and decrease of neurites
Abstract
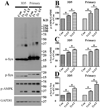
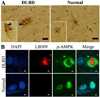
Neuronal inclusions of α-synuclein (α-syn), termed Lewy bodies, are a hallmark of Parkinson disease (PD). Increased α-syn levels can occur in brains of aging human and neurotoxin-treated mice. Because previous studies have shown increased brain lactate levels in aging brains, in PD affected subjects when compared with age-matched controls, and in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP), we tested the effects of lactate exposure on α-syn in a cell-based study. We demonstrated that (1) lactate treatment led to α-syn accumulation and oligomerization in a time- and concentration-dependent manner; (2) such alterations were mediated via adenosine monophosphate-activated protein kinase (AMPK) and associated with increasing cytoplasmic phosphorylated AMPK levels; (3) AMPK activation facilitated α-syn accumulation and phosphorylation; (4) lactate treatment or overexpression of the active form of AMPK decreased α-syn turnover and neurite outgrowth; and (5) Lewy body-bearing neurons displayed abnormal cytoplasmic distribution of phosphorylated AMPK, which normally is located in nuclei. Together, our results suggest that chronic neuronal accumulation of α-syn induced by lactate-triggered AMPK activation in aging brains might be a novel mechanism underlying α-synucleinopathies in PD and related disorders.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors have no actual or potential conflicts of interest. All animal work was approved by the Institutional IACUC.
Figures
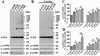







References
-
- Andersen AH, Zhang Z, Zhang M, Gash DM, Avison MJ. Age-associated changes in rhesus CNS composition identified by MRI. Brain Res. 1999;829:90–98. - PubMed
-
- Calne DB, Langston JW. Aetiology of Parkinson's disease. Lancet. 1983;2(8365-66):1457–1459. - PubMed
-
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

