Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review
- PMID: 23200525
- PMCID: PMC4131859
- DOI: 10.1016/j.biopsych.2012.09.031
Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review
Abstract
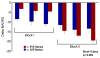
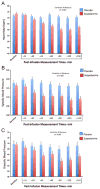
The muscarinic cholinergic receptor system has been implicated in the pathophysiology of depression, with physiological evidence indicating this system is overactive or hyperresponsive in depression and with genetic evidence showing that variation in genes coding for receptors within this system are associated with higher risk for depression. In studies aimed at assessing whether a reduction in muscarinic cholinergic receptor function would improve depressive symptoms, the muscarinic receptor antagonist scopolamine manifested antidepressant effects that were robust and rapid relative to conventional pharmacotherapies. Here, we review the data from a series of randomized, double-blind, placebo-controlled studies involving subjects with unipolar or bipolar depression treated with parenteral doses of scopolamine. The onset and duration of the antidepressant response are considered in light of scopolamine's pharmacokinetic properties and an emerging literature that characterizes scopolamine's effects on neurobiological systems beyond the cholinergic system that appear relevant to the neurobiology of mood disorders. Scopolamine infused at 4.0 μg/kg intravenously produced robust antidepressant effects versus placebo, which were evident within 3 days after the initial infusion. Placebo-adjusted remission rates were 56% and 45% for the initial and subsequent replication studies, respectively. While effective in male and female subjects, the change in depression ratings was greater in female subjects. Clinical improvement persisted more than 2 weeks following the final infusion. The timing and persistence of the antidepressant response to scopolamine suggest a mechanism beyond that of direct muscarinic cholinergic antagonism. These temporal relationships suggest that scopolamine-induced changes in gene expression and synaptic plasticity may confer the therapeutic mechanism.
Published by Elsevier Inc.
Figures
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
-
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–377. - PubMed
-
- Shelton RC, Loosen PT. Sleep deprivation accelerates the response to nortriptyline. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:113–123. - PubMed
-
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

