FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression
- PMID: 23200853
- PMCID: PMC3534912
- DOI: 10.1016/j.celrep.2012.10.021
FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression
Abstract
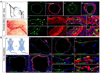
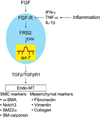
Maintenance of normal endothelial function is critical to various aspects of blood vessel function, but its regulation is poorly understood. In this study, we show that disruption of baseline fibroblast growth factor (FGF) signaling to the endothelium leads to a dramatic reduction in let-7 miRNA levels that, in turn, increases expression of transforming growth factor (TGF)-β ligands and receptors and activation of TGF-β signaling, leading to endothelial-to-mesenchymal transition (Endo-MT). We also find that Endo-MT is an important driver of neointima formation in a murine transplant arteriopathy model and in rejection of human transplant lesions. The decline in endothelial FGF signaling input is due to the appearance of an FGF resistance state that is characterized by inflammation-dependent reduction in expression and activation of key components of the FGF signaling cascade. These results establish FGF signaling as a critical factor in maintenance of endothelial homeostasis and point to an unexpected role of Endo-MT in vascular pathology.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nature Medicine. 2004;10:382–388. - PubMed
-
- Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. American journal of physiology Renal physiology. 2011;300:F721–F733. - PMC - PubMed
-
- Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi M, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karasan A. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Developmental cell. 2011;21:288–300. - PubMed
-
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

