Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis
- PMID: 23200863
- PMCID: PMC3516598
- DOI: 10.1016/j.ajhg.2012.10.024
Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis
Abstract
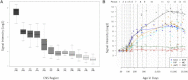
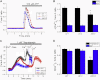
In this study, we combined linkage analysis with whole-exome sequencing of two individuals to identify candidate causal variants in a moderately-sized UK kindred exhibiting autosomal-dominant inheritance of craniocervical dystonia. Subsequent screening of these candidate causal variants in a large number of familial and sporadic cases of cervical dystonia led to the identification of a total of six putatively pathogenic mutations in ANO3, a gene encoding a predicted Ca(2+)-gated chloride channel that we show to be highly expressed in the striatum. Functional studies using Ca(2+) imaging in case and control fibroblasts demonstrated clear abnormalities in endoplasmic-reticulum-dependent Ca(2+) signaling. We conclude that mutations in ANO3 are a cause of autosomal-dominant craniocervical dystonia. The locus DYT23 has been reserved as a synonym for this gene. The implication of an ion channel in the pathogenesis of dystonia provides insights into an alternative mechanism that opens fresh avenues for further research.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Velickovic M., Benabou R., Brin M.F. Cervical dystonia pathophysiology and treatment options. Drugs. 2001;61:1921–1943. - PubMed
-
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group A prevalence study of primary dystonia in eight European countries. J. Neurol. 2000;247:787–792. - PubMed
-
- Duffey P.O., Butler A.G., Hawthorne M.R., Barnes M.P. The epidemiology of the primary dystonias in the north of England. Adv. Neurol. 1998;78:121–125. - PubMed
-
- Skogseid I.M., Malt U.F., Røislien J., Kerty E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur. J. Neurol. 2007;14:1129–1137. - PubMed
-
- Chan J., Brin M.F., Fahn S. Idiopathic cervical dystonia: Clinical characteristics. Mov. Disord. 1991;6:119–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

